
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:OF
Be
OB
Be
F
Transcribed Image Text:Question 17 of 40
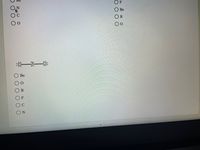
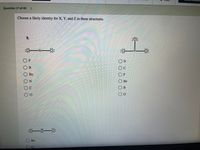
Choose a likely identity for X, Y, and Z in these structures.
:i:
В
Ве
F
О Ве
C
:一一:
Be
O O O O O O
O0O0 OO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5. Do the following structures represent resonance structures? If yes, identify them by stating the more stable and less stable. ? C=0 a) н H. CH2 H H ? H-CE H. d) Harrow_forwardDetermining HB or notarrow_forwardWhich of the following pairs are feasible resonance structures? I. CH₂=CHCH₂ and II. III. IV. V. III IV CH₂=CH :OH CH3-C-H :0: CH3-C-H and and and CH,−O−CH, and CH3-C- || :Ö: CH₂-C-H CH3-C-H CH₂CH₂OH -Harrow_forward
- Number 60arrow_forwardMInbox (5) - bubbatx1998@gmail.cx A ALEKS Daniel Estrella - Pre-Clas X bAnswered: NAverage Bond Entha My Drive - Google Drive X X X www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-lgNslkr7j8P3jH -IBiKFdvJdkR4Qg D Fu FbLE9J HQJOYSYezxnZjy7ErUilWNytDwYCaGIl7ay3s981 NTPxuxnt8g-2ai_6UhWKeYIDgamBWqio?1oBw7QYjlbavbS... AMy Application Stat... IT Welcome Aggie C... Hazlewood Act- Te... New Tab a Amazon.com: Gene... AVeterans - Addition... T Aggie Transition Ca... StudentLoans.gov |... Apps http://bookshelf.vi... Pre-Class Quiz #17- 10/11/19 Question 3 of 3 (1 point) Daniel 2 = 3 = 1 (H,0). If 0.540 g Aqueous hydrochloric acid (HCI) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium chloride (NaCl) and liquid water of sodium chloride is produced from the reaction of 0.73 g of hydrochloric acid and 0.56 g of sodium hydroxide, calculate the percent yield of sodium chloride. dlo Be sure your answer has the correct number of significant digits in it. ? X Submit Assignment 2019…arrow_forwardHow many o bonds are in the structure of the organic molecule below? H ***** :O: -N: C Tap here or pull up for additional resources :0: IC :Z—H N Н C 010 с N: Z: H N Question 8 of 18 C-Harrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY