
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Complete the following noting structures for A, B, and C. If more than one step is required to list them as 1, 2, 3, and so on.
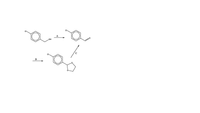
Transcribed Image Text:CI
A
HO
CI
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider each pair of structures below. Indicate the isomeric relationship between each pair. Your choices are (i) identical structures (ii) constitutional isomers, (iii) conformational isomers, (iv) configurational isomers or (v) resonance structures.arrow_forwardIdentify and describe mistake(s) in the following structures.arrow_forwardGive detailed Solution with explanation needed with structure numbering. Don't use Ai for answering this..Give IUPAC name for the given compoundarrow_forward
- Rank the three compounds below in order of shortest to longest carbon–oxygen bond length and explain your reasoning A)Tetrahydro-2H-pyran-2-one B)cyclohexane C)1-Methyl-2-piperidinonearrow_forwardWe consider the following molecule A: And. THIS Of the following Newman projections, indicate: MeEt F F Me 1 Br 5 Br F And Me THIS CH2CH3 AT CH2CH3 "CH3 Br Br Me Me. THIS 2 Br 6 And And .And And Br CI Me F And 3 F And 7 Me the one that corresponds to the least stable conformation of molecule A: And Br And the one that corresponds to the most stable eclipsed conformation of molecule A: CI Me THIS And Br And Br F F And Me And Choose... Choose... the one that corresponds to molecule A as seen by the eye of the observer in the statement: Choose... the one that corresponds to the least stable staggered conformation of molecule A: the one that corresponds to the most stable conformation of molecule A: Choose... Choose... ◆arrow_forwardThe structure of hydroxymethylcyclohexane is shown below. OH Given this structure, answer the following: Part 1: How many degrees of unsaturation (index of hydrogen deficiency) does this molecule have? (Calculate its IHD.) Part 2: There are five constitutional isomers of hydroxymethylcyclohexane that contain the cyclohexane ring. Draw these below in the box provided.arrow_forward
- 4G 4G 1:54 PM 43 ... PROBLEM SET –- Organic Nitrogen Compounds 1. Classify each of the following compounds as either a primary, secondary, or tertiary amine: (a) CH3 (c) CH3 N-H CH;CH,CHNH2 CH3arrow_forwardGive detailed Solution with explanation needed. Don't give Handwritten answerarrow_forwardWhat is the conformation of ring A? of ring B? of ring C? of ring D?arrow_forward
- Provide the IUPAC name for this structure: Type your answer and submit X₂ X² Ω· Write your response here.…... ОН ОНarrow_forwardOrganic ChemistryFor each pair of compounds, indicate whether the first structure is lower, higher, or about the same in energy as the second structure. Be sure to explain. C angle (c) CH3 OH is OH in energy than/as H3C because: Po (d) CN CN NC- CN CO₂Et is in energy than/as CO₂Et CO₂Et EtO₂C because:arrow_forwardA 1,2-cis disubstituted cyclohexane, such as cis 1,2-dichlorocyclohexane, must have one group axial and one group equatorial. Explain,arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY