
Identify all electrophilic sites in chloroacetyl chloride. Given the (expected) result of the first step of the synthetic sequence, which of these sites is more electrophilic? Does this result make sense, given what you know about the relationship between structure and reactivity?
ATTACHED IS THE REACTION WITH THE REACTION PROCEDURE BELOW
PROCEDURE
To a 125 mL Erlenmeyer flask containing a magnetic stirbar was added 2,6-dimethylaniline (1, 3.0 mL), followed by acetic acid (20 mL). The mixture was stirred until homogeneous and then chloroacetyl chloride (2.0 mL) was added in one portion. Immediately after the addition of chloroacetyl chloride, a concentrated solution of sodium acetate (25 mL) was added in one portion. The mixture was stirred for about five minutes and then DI water (65 mL) was added directly to the reaction mixture in one portion.
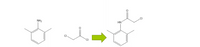
Step by stepSolved in 2 steps with 1 images

- 3.11: The enzyme catalyzing the reaction below, thought to participate in the fermentation of lysine in bacteria, was recently identified and characterized (J. Biol. Chem. 2011, 286, 27399). Propose a likely chanism. Hint: the mechanism involves two separate carbon-carbon bond forming and bond breaking steps. C of acetyl CoAis identified with a red dot to help you trace it through to the product. NH3 0 NH3 0 co H3C .CO2 SCOA SCOAarrow_forward15.6 (pqr) Predict the major product or reagent that completes the transformationarrow_forwardWrite structures for the carbonyl electrophile and enolate nucleophile that react to give the enone below. مثله You do not have to consider stereochemistry. . Draw the enolate nucleophile in its carbanion form. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down menu. سكر ٥٠ n ChemDoodle 4.arrow_forward
- Please see attachedarrow_forwardDraw structural formulas for both resonance structures of the enolate ion obtained by treating the carbonyl compound below with base. • You do not have to consider stereochemistry. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate resonance structures using the → symbol from the drop-down menu. Submit Answer ChemDoodle Retry Entire Group 9 more group attempts remainingarrow_forward2. In Experiment 4B, it was shown from the NMR spectrum that the meta-di-substituted Nitration product had been recorded as a major product: COOME COOME HNO3 H,SO, NO2 Major product However, there were two other possible but less favorable routes, i.e. ortho- or para- positions for the nitronium to attack the aromatic ring with. Draw the H-NMR spectrum for Methyl p-Nitrobenzoate in case the substitution takes place at the para-position. Pay attention to the chemical shift, the splitting pattern, and approximate the integration in your spectra. Methyl p-Nitrobenzoate 3.0 2.5 2.0 1.5 - 1.0- 0.5 4 3. 2 1. 10 6. 8.arrow_forward
- #4,5,6arrow_forward4 pts total) re is Aromatic (A), (circle one) A AAN ion with Cl₂/FeCls. (6 pts) acids shown to right) (6 pts) al 3 1. Consider the Friedel-Crafts (FC) alkylation of N-phenylacetamide (shown below) in which reaction with 1-chloropropane and AlCl3 produces two isomers of isopropyl N-phenylacetamide. (20 pts) 1. Draw the complete reaction mechanism for the formation of the ortho product (para is also formed) (10 pts) 2. Is this reaction faster or slower than the similar FC reaction starting with benzene (C6H6)? Explain why. (4 pts) 3. Explain why this reaction produces the ortho and para products (but not meta). (6 pts) Use your mechanism to help explain parts #2 and #3. Use additional drawings and words as needed. to AICI N-phenylacetamide 455 sta AHO O 00 10H СОИН 08H НИ MOHO HO HO 10arrow_forwardDraw structural formulas for both resonance structures of the enolate ion obtained by treating the carbonyl compound below with base. You do not have to consider stereochemistry. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate resonance structures using the symbol from the drop-down menu. H CH₂ H H ? [ ] درarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





