
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please see attached
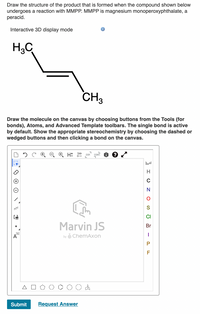
Transcribed Image Text:**Title: Understanding Peracid Reactions with MMPP**
**Objective:**
Learn how to draw the product structure formed when a compound undergoes a reaction with MMPP (magnesium monoperoxyphthalate), a peracid.
**Interactive 3D Display Mode:**
Visualize chemical structures in an interactive 3D environment for a better understanding of molecular geometry.
**Chemical Structure:**
- The image shows a hydrocarbon structure with two methyl groups (CH₃) on either end.
- Explore how this structure reacts with MMPP to form epoxides or other oxidized products.
**Drawing Instructions:**
1. **Tool Selection**: Use the canvas tools to select bonds, atoms, and advanced templates for structure drawing.
2. **Bond Activation**: The single bond is activated by default for ease of use.
3. **Stereochemistry**: Show appropriate stereochemistry by choosing dashed or wedged bonds, then click a bond on the canvas.
**Diagram Explanation:**
The diagram includes a drawing canvas provided by Marvin JS, featuring:
- **Toolbar**: Options for drawing and editing molecules.
- **Element Selection**: Sidebar with elements like H, C, N, O, S, Cl, Br, I, and F for easy access.
- **Drawing Shapes**: Basic molecular frameworks such as polygons and other shapes to facilitate drawing complex molecules.
**Application:**
Use this tool to practice drawing the reaction products of hydrocarbons with MMPP, reinforcing concepts learned in organic chemistry related to oxidation reactions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Briefly discuss emulsion and suspension polymerization techniques.arrow_forwardPicture attached belowarrow_forwardConsider each of the following monomers and predict if it would likely undergo radical, anionic, or cationic addition polymerisation. Then, in the space provided below, draw the electron pushing mechanism for the initiation and the first propagation steps of the monomer that you chose for each (the catalyst is provided for you). Then draw what the growing polymer chain looks like and briefly indicate why you chose that monomer to undergo the selected type of polymerisation (hint: consider concepts such as electron donating group, electron withdrawing group, resonance, and hyperconjugation!)arrow_forward
- Molecular weight of a condensation polymer hardly increases if the reaction is stopped either at 45% conversion or at 80% conversion, respectively. - Explainarrow_forwardBased on the answer to my question my follow-up question refers to processing. How does hydrogen bonding interactions affect the processing temperatures of these polymers? And what would we expect to occur? Why does hydrogen bonding affect thermal stabiltiy (thermo-oxidative stability) and affect degradation of the macromolecule. What is the mechanism of this interaction?arrow_forwardConsider each of the following monomers and predict if it would likely undergo radical, anionic, or cationic addition polymerisation. Then, in the space provided below, draw the electron pushing mechanism for the initiation and the first propagation steps of the monomer that you chose for each (the catalyst is provided for you). Then draw what the growing polymer chain looks like and briefly indicate why you chose that monomer to undergo the selected type of polymerisation (hint: consider concepts such as electron donating group, electron withdrawing group, resonance, and hyperconjugation!)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY