
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
for example 6.11 please explain what the reagents do and write out step by step how they change the molecules a and b
Transcribed Image Text:a
ate buffer
hols
HCIOg
hlorous acid
11
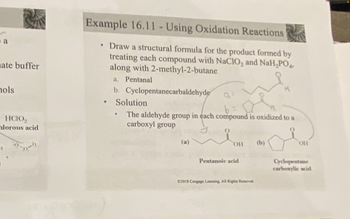
Example 16.11 -Using Oxidation Reactions
Draw a structural formula for the product formed by
treating each compound with NaClO₂ and NaH₂PO4)
along with 2-methyl-2-butane
a. Pentanal
b. Cyclopentanecarbaldehyde
Solution
b
The aldehyde group in each compound is oxidized to a
carboxyl group
(a)
OH
Pentanoic acid
C2018 Cengage Learning. All Rights Reserved.
(b)
H
OH
Cyclopentane
carboxylic acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following correctly describes the known mechanism of the Diels-Alder reaction? Select one: A. A strained 4-member ring intermediate is formed which rearranges to a 6- member ring. B. A di-radical, 6-member ring intermediate is formed. C. The mechanism is a one-step process with bond-making, bond breaking changes occurring simultaneously. D. A zwitterion species (molecule with full +1 and -1 charges) is rapidly formed as an intermediate. E. A resonance-stabilized carbocation intermediate is formed in a slow step.arrow_forward6. Write "most" under the member of each trio which is most stable. Write "least" under the member of each trio which is least stable.. a) C b) Ľarrow_forward[References] Using the Bronsted-Lowry model, write an equation to show why the following species produce a basic aqueous solution. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) HPO4 (aq) + H,0() = Submit Answer Try Another Version 5 item attempts remaining Visited 74 F Watcharrow_forward
- Solve the parts of 11.7arrow_forward3. Provide at least one significant alternative resonance form for each molecule below. Generate a total of six correct structures to receive credit for this question. Provide curved arrows on the given structure to show how it relates to the first structure you draw in each case. An example is shown below in blue. A. R2N° C. R2N. E. B. D.arrow_forward9. The two molecules shown below are structurally very similar. Draw all resonance structure for each species with curved arrows and circle which is molecule (A or B) is more stable because it has more resonance structures. Only use patterns 2 and 3. A Barrow_forward
- The photo dimerization of benzophenone to benzopinacol is initiated by what type of electronic transition that then rapidly decomposes to a diradical since putting in electrons in anti bonds breaks bonds! The diradical then starts abstracting hydrogens from solution as pictured in the text? σ = electrons in sigma bonds n = electrons in non-bonding orbitals π electrons in pi bonds. anti bonds Hint: This process is very important because although the molecule responsible for human vision (retinal) is not a very long conjugated pi system, this transition allows retinal to absorb visible light. a. n to σ* b. σ to σ* C. π το π d. n to П e. σ to П* f. π to σ'arrow_forwardConsider the bonds labelled A and B below. Which one has a higher bond strength and why? Comment on the answers of at least two of your course mates regarding whether you agree with their answers or not. Provide your reasoning in each case. Br Aarrow_forwardThat answer is incorrect. Is there another possible solution? Preferably in a cyclopentane shapearrow_forward
- .) Draw other reasonable resonance forms for the following structures. Circle the best resonance form for each structure. 4. a. b. NH₂arrow_forwardTutored Practice Problem 6.3.3 COUNTS TOWARDS GRADE Use formal charge and electronegativity to identify best resonance structure. Close Proble Three inequivalent resonance structures for carbonyl sulfide, SCO, are shown below, Assign formal charges to all atoms in the resonance structures and identify the more likely resonance structure. Note: If a row is not needed, leave it blank. 5-c = ö :S=C - ö: :S-C = 0: A B The more likely resonance structure for SCO is [ v Check & Submit Answer Show Approacharrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY