
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:X Montana Tech Montana's Prem X M Montana Tech
https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-lg
->
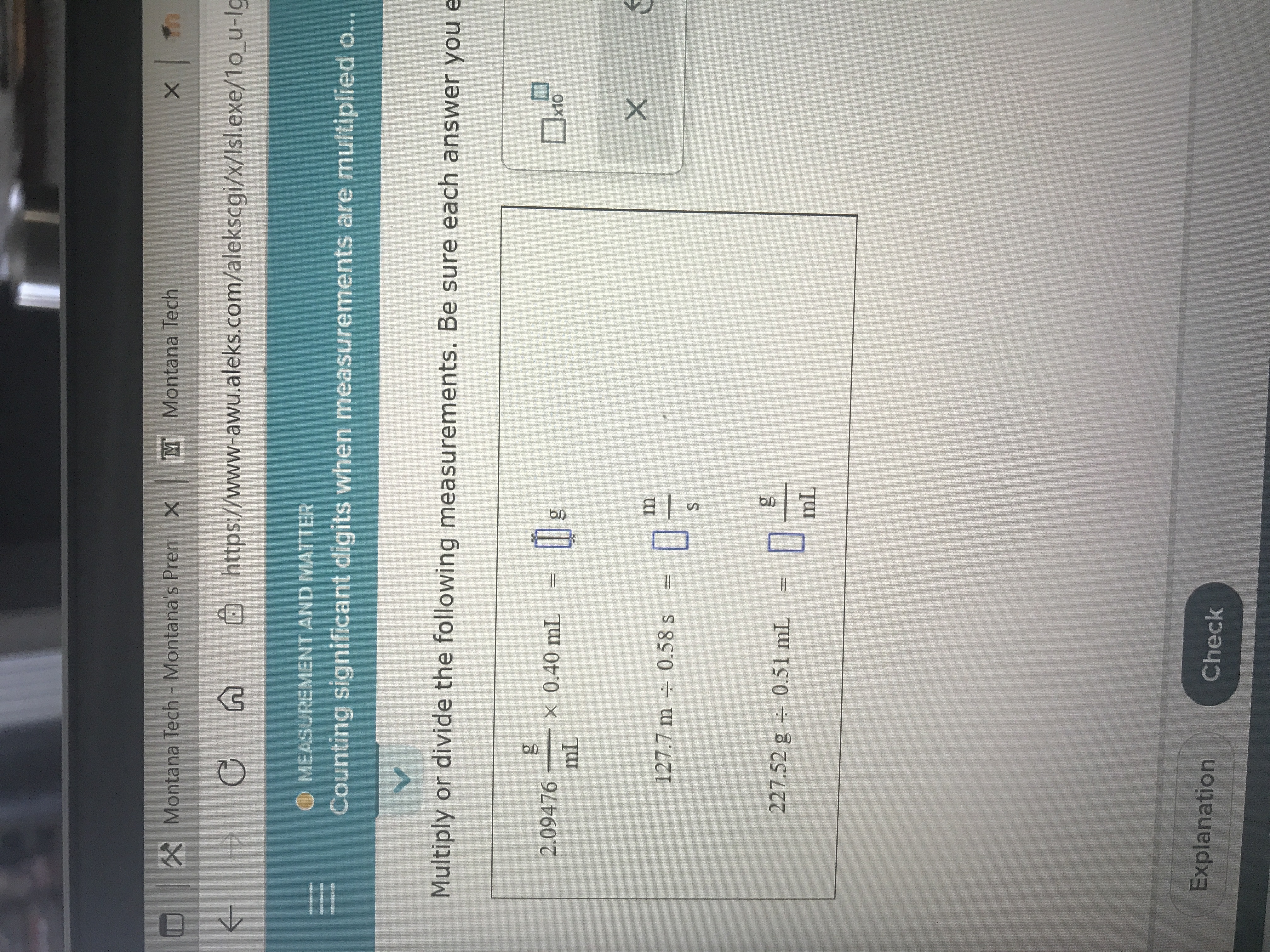
O MEASUREMENT AND MATTER
Counting significant digits when measurements are multiplied o...
Multiply or divide the following measurements. Be sure each answer you e
2.09476
X 0.40 mL
m
127.7 m + 0.58 s
S.
227.52 g 0.51 mL = |
Explanation
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!!!!!!!arrow_forward10) Solve each problem and report your answer to correct significant figure and units. Remember that units multiply, divide, add, subtract and simplify just like variables. ml 65.0 kg + 0.05 kg + 179.1 kg = 17 g x 1.08 175 cm - 22.5 cm = 9.20 g/ (1.72 cm³ + 21 cm³) = ot avlo2a 3.23 x 10=23 ma (23.0 lb/gal)(2 ft) = (2.1 x 10-10 cm)3 to 1edmun sdi enimale0 (2.23 x 103 g)(9.0 × 10-3 g) (3.1 x 104 nm²)1/2 = Jmy to. 10.1 x 10-7 s2arrow_forwardanswer in proper number of significant figures. 1. Convert 6.4 decades to seconds. 10 years)x(30 (24 hours 60 minuies 60 seconds I minatte 6.4 decades x 305 dags I decade I year I dag 1 hour 2. Convert 71.0 Pa to dyne/cm?. 3. It was found out that 2.0 kg of sucrose can dissolve per liter of water at 25°C. How many tablespoons of water is needed to dissolve 54 g of sucrose?arrow_forward
- A sample of gaseous neon atoms at atmospheric pressure and 0 degrees C contains 2.69*10^22 atoms per liter. The atomic radius of neon is 69 pm. What fraction of the space is occupied by the atoms themselves? Express with 2 significant figures.arrow_forward口 A www-awu.aleks.com S Zoom A ALEKS - Evelin Vazquez - Learn A Start Page * Start Page O MEASUREMENT AND MATTER Calculating mass density liquid density methyl acetate 0.93 mL g 0.63 mL pentane 0.79 mL acetone g 0.89 mL tetrahydrofuran g 1.6 mL carbon tetrachloride Next, the chemist measures the volume of the unknown liquid as 1925. cm° and the mass of the unknown liquid as 1.80 kg. Calculate the density of the liquid. Round your n, / mL answer to 3 significant digits. O yes Given the data above, is it possible to identify the liquid? O no O methyl acetate pentane acetone If it is possible to identify the liquid, do so. tetrahydrofuran O carbon tetrachloride Explanation Check O 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use 1,789 SEP stv 29arrow_forwardQuestion in picturearrow_forward
- How do I solve the following problem with the proper number of significant figures? Mainly parts E and F.arrow_forwardThat wasn't the answer im afraid.arrow_forwardCarbon monoxide(CO) is a poisonous gas because it binds..... calculate the volume in the listers occupied by a carbon monoxide in a room that measures 19.11m long, 11.71 m wide, 16.09 m high at this concentration. Be sure your answer has the correct number of significant digits. Volume of CO in the room: Larrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY