
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
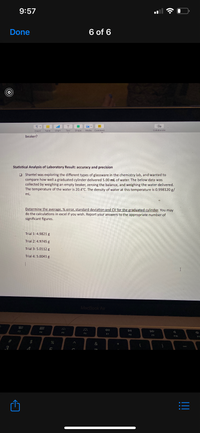
Transcribed Image Text:**Statistical Analysis of Laboratory Result: Accuracy and Precision**
Shantel was exploring the different types of glassware in the chemistry lab and wanted to compare how well a graduated cylinder delivered 5.00 mL of water. The below data was collected by weighing an empty beaker, zeroing the balance, and weighing the water delivered. The temperature of the water is 20.4°C. The density of water at this temperature is 0.998120 g/mL.
**Objective:**
Determine the average, % error, standard deviation, and coefficient of variation (CV) for the graduated cylinder. You may do the calculations in Excel if you wish. Report your answers to the appropriate number of significant figures.
**Data from Trials:**
- Trial 1: 4.9821 g
- Trial 2: 4.9745 g
- Trial 3: 5.0112 g
- Trial 4: 5.0041 g
Expert Solution
arrow_forward
Step 1
Given information:
Trial 1: 4.9821 g
Trial 2: 4.9745 g
Trial 3: 5.0112 g
Trial 4: 5.0041 g
Density of water = 0.998120 g/mL
Step by stepSolved in 5 steps with 1 images

Knowledge Booster
Similar questions
- After finishing the lab, Peter successfully identified his unknown was acetone (molar mass: 58.1 g/mol). He then realized that he had forgotten to write down the temperature on his data sheet because he and has partner had done the math together. Peter unfortunately doesn't know his lab mates phone number or last name, so he can't look him up on social media. Poor Peter - he only has 30 minutes to turn in his lab report. Based on the data he collected below - what was the temperature of his water bath? Peter remembers that his water bath never boiled, but his acetone did fully evaporate. Mass of flask: 77.002 g Mass of flask + unknown condensed liquid: 77.487 g Temperature of water bath: ???? Pressure of gas in our system: 0.997 atm Volume of gas in our system: 250 mL Group of answer choices: A) 85 °C B) 100 °C C) 91 °C D) 97 °Carrow_forwardPlease help with question 3arrow_forwardVolumetric Glassware and Balances In an experiment for calibration of a 5.00 mL. pipet, the following data was obtained for 5.00 ml. of the pipet at 15°C The correction <--0.04 mL The volume of 1 g of water at 15°C- 1.0021 mL The weight of water delivered by the pipet (g) is: a) 5.06 b) 4.92 c) 5.03 d) 4.95 e) 5.09arrow_forward
- Part A Solve for x, where M is molar and s is seconds. (2.7 x 103 M-2s¬1)(0.22 M)³ Enter the answer. Include units. Use the exponent key above the answer box to indicate any exponent on your units. View Available Hint(s) HA Value Units Submit Previous Answers Request Answer X Incorrect; Try Again; 3 attempts remainingarrow_forwardYour window thermometer shows that the temperature outdoors is 84.1°F. How should you report this temperature to your friend in Israel (i.e., on the Celsius scale) and to your SI-enthusiastic physicist neighbor (i.e., on the Kelvin scale)? temperature in degrees Celsius: temperature in kelvins: Karrow_forwardThe mass of a beaker is 8.342 grams. After 3.00 ml. of an organic liquid is pipetted into the beaker. the combined mass of the beaker and the organic liquid is 10.697 grams. From the dat, what is the measured density of the organic liquid ?arrow_forward
- Two students perform the analysis of an alloy containing aluminum and zinc by the same method that we used in Experiment 2. They obtained the following data 0.007 g Mass of empty gelatin capsule Mass of gelatin capsule plus alloy sample 0.336 g 141.209 g Mass of empty beaker Mass of beaker plus displaced water 307.712 g 20 °C Temperature of water (and reaction mixture) Barometric pressure Density of water at 20°C 29.32 in. Hg 0.09982 g/mL 17.5 mm Hg Vapor pressure of water at 20°C What is n ? H₂ -2 a. 3.103 x 10 b. 6.336 x 10 c. 4.442 x 10 d. 3.030 x 10 e. 1.089 x 10 -1 -2arrow_forwardC The Average Adult Human Burr x b Search results for 'Convert 3.2 x A app.101edu.co Email YouTube A Maps 101 Chem101 Update EReading Question 3 of 23 Subr The average adult human burns 2.00 x 10 kcal per day in energy. What is this rate in kJ per hour ? MOUNT ADD FACTOR ANSWER RESET 24 60 4184 2.01 x 106 4.184 1.15 x 10$ 1 0.001 20.0 349 2.00 x 10 1000 kcal/day days kJ cal kJ/hr hr min kcal %24arrow_forward▾ Part A DA chemical reaction does not occur for this question When ethanol (C₂HO(aq)) is consumed, it reacts with oxygen gas (O₂) in the body to produce gaseous carbon dioxide and liquid water. Enter the balanced chemical equation for the reaction Express your answer as a chemical equation including phases. | ΑΣΦ Submit Previous Answers Request Answer x Incorrect: Try Again; 6 attempts remaining SARASER E Provide Feedback MAIKLIENT Next >arrow_forward
- Coal tar lotion containing 5% coal tar is needed for one of your patients. There is 100ml of 2% coal tar lotion in stock. What quantity (in grams) of coal tar is required to be added to this 2% w/v coal tar lotion to provide the 5% coal tar lotion prescribed? (Give your answer to one decimal place). units - garrow_forwardConsider the following graph: mass (g) 100 80 60 10 (11.806, 95.415) Linear Fit for: Data Set | mass mass=mx+b m (Slope): 3.60 g/mL b (Y-Intercept): 11.4 g Correlation: 0.946 RMSE: 8.50 g 15 volume (mL) 20 In the graph shown for the problem, what is the variable and units on the independent axis? What is the variable and units on the dependent axis? 25arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY