Complete the biosynthetic pathway for the synthesis of Cadinene. Hint: Cyclize to form a ten membered ring and then do a 1-3 hydride shift. Cyclize again and loose a proton. CH3 CH3 CH3 CH3 Eudesmol CH3 ОPP OH H3C CH3 CH2 Cadinene
Complete the biosynthetic pathway for the synthesis of Cadinene. Hint: Cyclize to form a ten membered ring and then do a 1-3 hydride shift. Cyclize again and loose a proton. CH3 CH3 CH3 CH3 Eudesmol CH3 ОPP OH H3C CH3 CH2 Cadinene
Oh no! Our experts couldn't answer your question.
Don't worry! We won't leave you hanging. Plus, we're giving you back one question for the inconvenience.
Submit your question and receive a step-by-step explanation from our experts in as fast as 30 minutes.
You have no more questions left.
Message from our expert:
Hi and thanks for your question! Unfortunately we cannot answer this particular question due to its complexity.
We've credited a question back to your account. Apologies for the inconvenience.
Your Question:
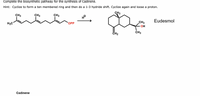
Transcribed Image Text:Complete the biosynthetic pathway for the synthesis of Cadinene.
Hint: Cyclize to form a ten membered ring and then do a 1-3 hydride shift. Cyclize again and loose a proton.
CH3
CH3
CH3
CH3
Eudesmol
CH3
ОPP
OH
H3C
CH3
CH2
Cadinene
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
