
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
how do i do this ?
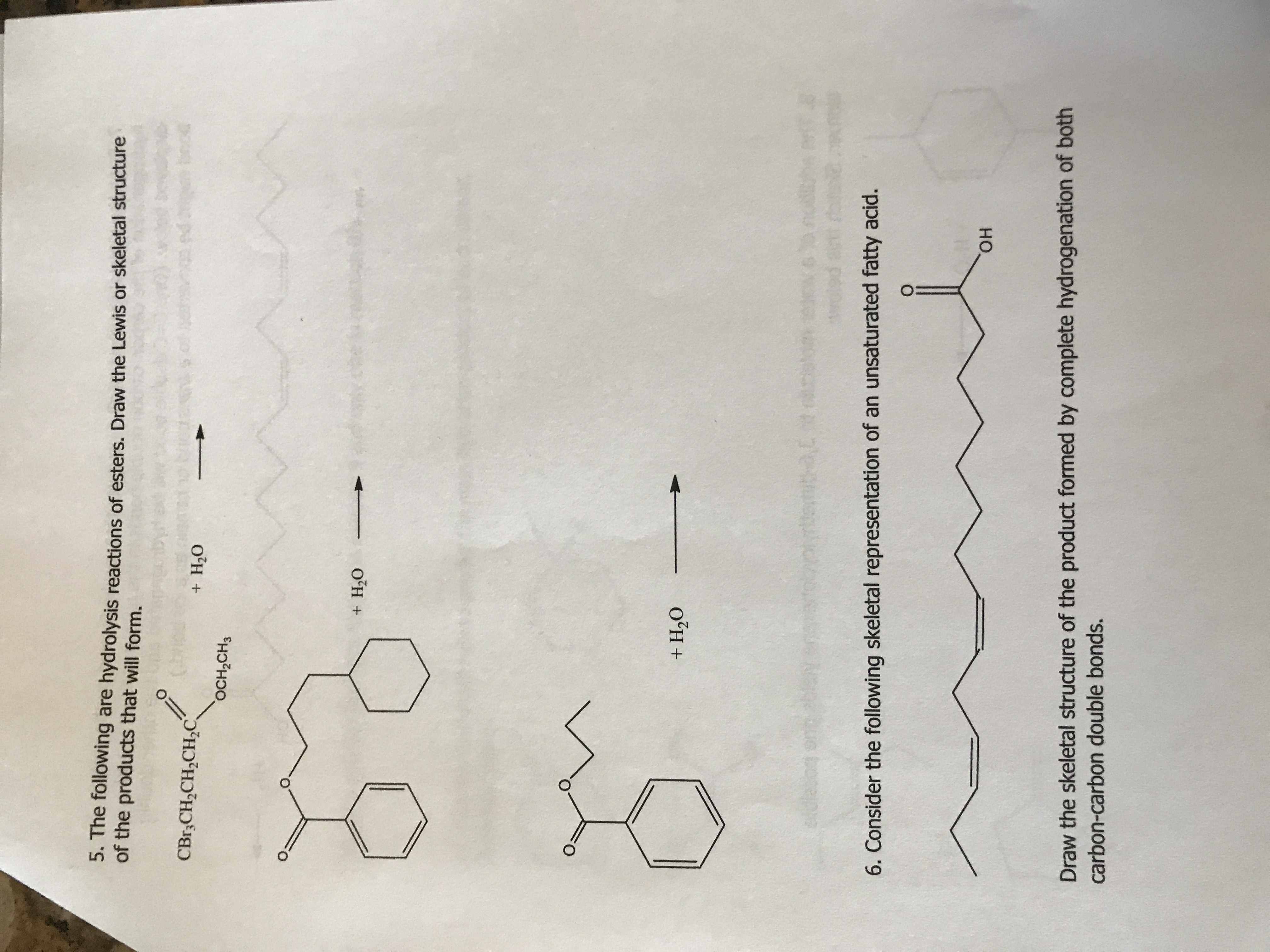
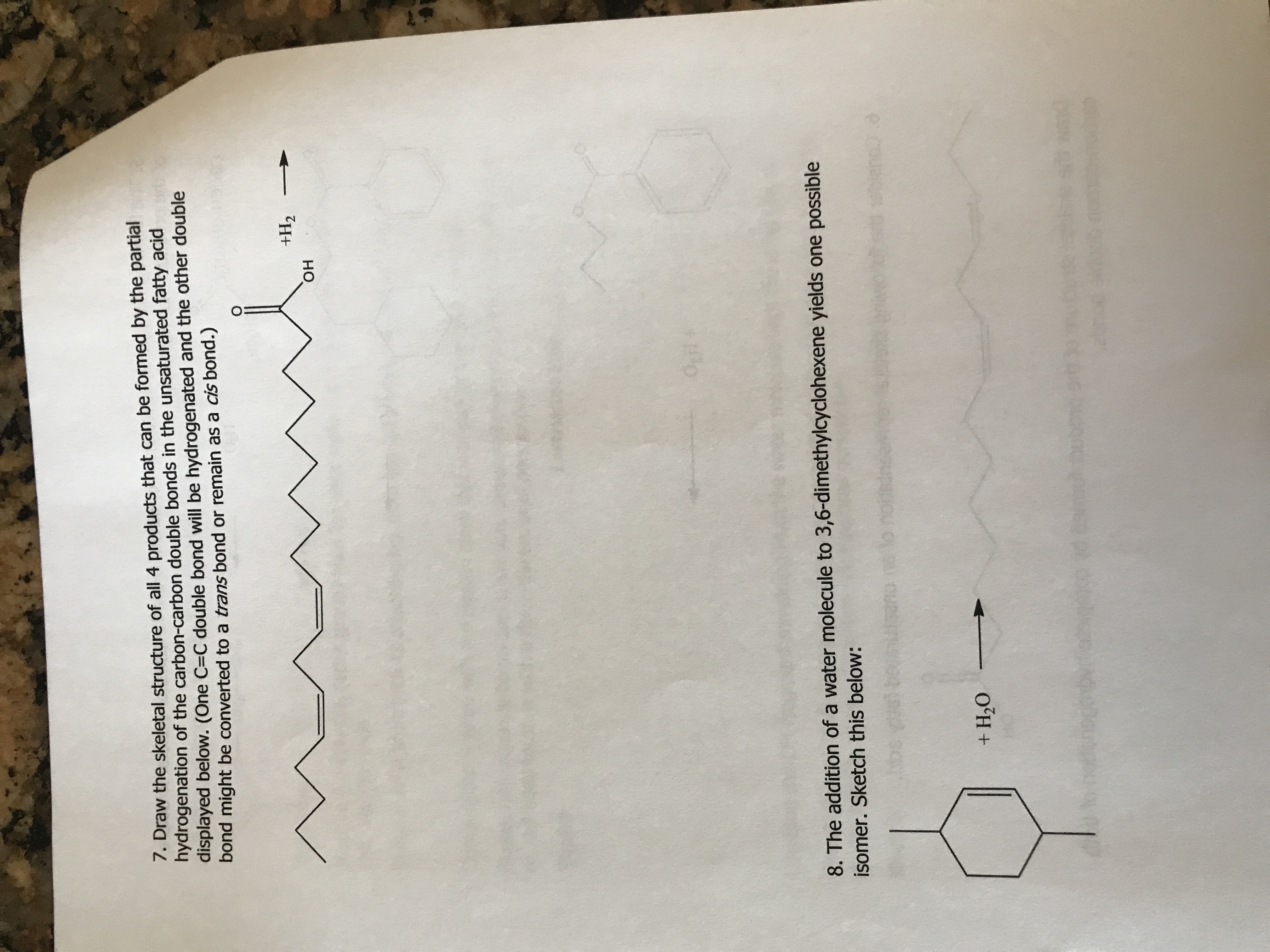
Expert Solution
arrow_forward
Step 1 Introduction
On hydrolysis, esters undergo cleavage to produce carboxylic acid and alcohol. The reaction occurs in the presence of an aqueous acid(H2SO4) or an aqueous base(NaOH).
The mechanism of base hydrolysis involves the following steps.
- The nucleophilic attack of carbonyl carbon by Hydroxyl ion(OH-) resulting in tetrahedral intermediate.
- The tetrahedral intermediate disintegrates by the release of the alkoxide group reforming carbonyl group.
- Thus, acid is produced from the tetrahedral intermediate, and the alkoxide group converts to alcohol.
The mechanism of acid hydrolysis involves the following steps.
- Protonation of ester carbonyl making it more electrophilic.
- Water oxygen acts as the nucleophile attacking the electrophilic C=O group forming a tetrahedral intermediate.
- Deprotonation of water group attached to the tetrahedral intermediate.
- Alkoxide group is made a good leaving group by protonation.
- Neutral methanol gets released.
- Deprotonation of oxonium ion results in a carboxylic acid.
NOTE: According to Bartleby guidelines, one question should be answered. I am answering Q5.
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following is a benefit of implementing a Total Productive Maintenance (TPM) program? a) Reduced downtime b) Increased production costs c) Higher inventory levels d) Longer lead timesarrow_forward16 part earrow_forwardJust answer question 15 show the step correct by step to step .arrow_forward
- Dopamine is commercially available in vials containing 400 mg in 20 mL. An order for 350 mg comes to the pharmacy. How many mL are needed to make this dose?arrow_forward____________ makes up the majority of oil use, with gasoline and diesel fuel being the most common fuels for this genre. __________ makes up the majority of natural gas usage. Electricity makes up the majority of coal burning. Fill in the Blank!arrow_forwardU guys gotta help me on this !!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY