
Chemistry In Focus
7th Edition
ISBN: 9781337399692
Author: Tro, Nivaldo J.
Publisher: Cengage Learning,
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
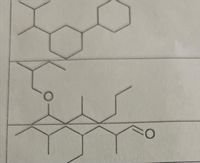
name these shapes.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict which will have the higher boiling point: ICl or Br2. Explain your reasoning.arrow_forwardThe melting and boiling points of sodium metal are much lower than those of sodium chloride. What does this difference reveal about the relative strengths of metallic bonds and ionic bonds?arrow_forwardName and draw and example of three basic ionic crystal structures.arrow_forward
- what is the difference between bonding forces holding compounds together and other attractive forces?arrow_forwardIdentify what type of intramolecular forces (Ionic, Covalent, Metallic) exist in the following compounds. Ferric oxide Potassium nitrate Methane Calcium chloridearrow_forwardExplain, in molecular terms, why oil and water do not mix.arrow_forward
- a) What types of elements are bonded together to make an ionic compound? Give three examples of ionic compounds. Identify the types of elements that make up the compound in each example. B) Describe how the physical state of a substance at room temperature depends on the strength of the intermolecular forces between the particles of the substance.arrow_forwardhow can you use the physical properties of melting solubility and conductivity to classify ionic and covalent compounds?arrow_forwardis sulfur tetrachloride polar or nonpolar?arrow_forward
- What is the VSEPR Shape of Water?arrow_forwardDescribe in detail the microscopic processes that take place when a liquid boils. What kind of forces must be overcome? Are any chemical bonds broken during these processes?arrow_forward2 4 5 6 7 For each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or between molecules of the compound and molecules of water. compound hydrogen-bonding force formula or Lewis Between molecules of the name Between molecules of structure the compound and molecules of water? compound? yes yes hydrogen iodide HI no no yes yes NH3 ammonia no no н :0: н yes yes Н—с —с-о — н no acetic acid no Н ? x 5 Submit 2019 McGraw-Hill Education. All Rights Reserved Terms o Continue MacBook Pro F.O F8arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning