
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
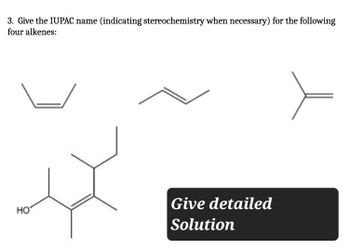
Transcribed Image Text:3. Give the IUPAC name (indicating stereochemistry when necessary) for the following
four alkenes:
HO
Give detailed
Solution
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- prepaer the follwing compoundsarrow_forward4. The enthalpies, AH°, for the hydrogenation of three alkenes are shown below: Alkene A: -50 kcal/mole Alkene B: -60 kcal/mole Alkene C: -70 kcal/mole Which alkene is the least stable and why?arrow_forwardB. Aliphatics and Alkyl Halides Name the following compounds. 2. CH3-CH₂-CH-CH3 1 I-C-CH3 | CH3arrow_forward
- (a) • Consider E/Z stereochemistry of alkenes. • Do not show stereochemistry in other cases. • You do not have to explicitly draw H atoms. Draw a structural formula for the alkene: 2-methyl-2-heptene. MUIL Templates Sn [F ? Ⓡ ChemDoodle (b) Draw a structural formula for the alkene: (E)-1-chloropropene.arrow_forward9. Read very carefully: Alkenes can be OXIDIZED to form carbonyl compounds (- C=0) (see example below). KNOWING this, how would you distinguish the following two alkenes by using this OXIDATIVE reaction? R-CH=CHCH3 à (oxidation) R -CO,H + CH;CO,H BOTH undergo OXIDATIONarrow_forwardGive the systematic (IUPAC) name for the following compounds. Include stereochemistry in the name ONLY FOR THOSE STRUCTURES THAT ALLOW YOU TO DETERMINE STEREOCHEMISTRY. In other words, describe the stereochemistry if the molecule is shown as a Fisher projection, or as a perspective formula, or if an alkene is drawn with the correct stereochemistry representationarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY