
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Annotate the Mass Spec, 1H NMR, and 13C NMR for C4H11N or Butylamine
Annotate the Mass Spec, 1H NMR, and 13C NMR for C4H8O or Butraldehyde
Annotate the Mass Spec, 1H NMR, and 13C NMR for C5H8O or Cyclopentanone
Thanks :)
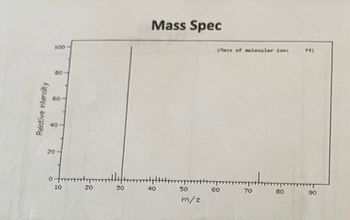
Transcribed Image Text:Relative Intensity
100-
60
40
20-
0-
10
20
30
Mass Spec
40
t
50
m/z
(as of molecular font
60
70
80
73)
90
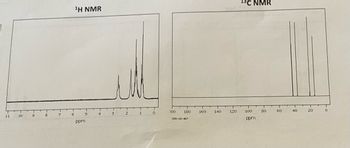
Transcribed Image Text:11
10
9
8
7
6
¹H NMR
5
ppm
4
2
0
200
180
COS-03-467
160
140
120
13C NMR
100
ppm
80
60
40
20
0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the splitting pattern and assign and label the H of the spectrum of C4H8Oarrow_forwardThe mass spectrum of Compound C is shown below: Draw the most likely species responsible for the signals at m/z 91.arrow_forward7) Chemical Formula: C₂H₁N IR: weak peak at 3400cm-1 7 1H SH 2H 8) Chemical Formula: C8H1204 IR: strong peak 1720cm-1, peak at 1650cm-1 Unknowns for 1H NMR Final Report 9) Chemical Formula: C5H1002 IR: strong peak at 1750cm-1 1H (8~27 = 16-+2 = € /2=2. PPM 4H 2H 3 PPM 3H 3H 2 * H - (8x2) = 16-12=412=2 -G-H H h+1= 2 4 6H 6Harrow_forward
- Determine the compound (name or structure) from the data. Explain features from each data. Molecular formula: C6H5Br Use molecular formula to determine IHD IR: Identify the presence/absence of five key functional groups NMR: Analyze this last. Consider multiplicity and peak area to confirm compound. The 5 H peak area covers both peaks (at 7.1 and 7.5 ppm Structure?arrow_forwardGiven the following molecule, draw its predicted standard spectrum along with DEPT 90 and DEPT 135 spectra. Then, based on that picture, assign the numbered carbons on the original structure to a peak in the standard 13C spectrum. Provide a brief explanation for your choices.arrow_forwardWhich functional group, except alkane, is presence in the following IR spectrum? INFRARED SPECTRUM Relative Transmittance 0.96 0.92 carboxylic acid ether alcohol alkene 3000 2000 Wavenumber (cm-1) m 1000arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY