
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
11thank u
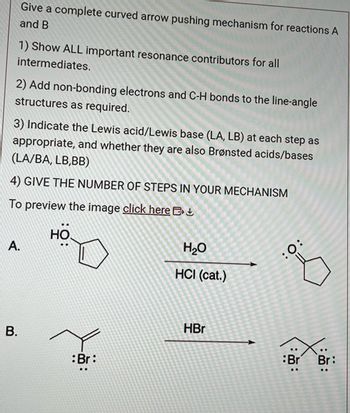
Transcribed Image Text:Give a complete curved arrow pushing mechanism for reactions A
and B
1) Show ALL important resonance contributors for all
intermediates.
2) Add non-bonding electrons and C-H bonds to the line-angle
structures as required.
3) Indicate the Lewis acid/Lewis base (LA, LB) at each step as
appropriate, and whether they are also Brønsted acids/bases
(LA/BA, LB,BB)
4) GIVE THE NUMBER OF STEPS IN YOUR MECHANISM
To preview the image click here
A.
B.
9:
:Br:
H₂O
HCI (cat.)
HBr
:O:
Br
:
Br:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. Convert 5.0 Tbytes to Mbytes. 5tutesx 0.000001 mby- ts - 5.00 0no mbytes 1 चि je %3D 1 fera 15.0x10 1. Subtract 3.58 x 102 kg from 1.41 x 101 ng and report your answer in g.arrow_forward4 $2004 АД eptarrow_forwardg. h. wit $ Ph Br li NaOCH, CH₂OH (cold, short time) 1. LDA 2 Br OCH₂ & 1. NaOCH₂ CH₂OH 2. BrCH₂CH₂CH₂ 3. H+, H₂O, heat 1. PPh 2. BuLi 3. NaOEt OEt EtOH NaOCH, CH₂OH (warmup, long time)arrow_forward
- 7arrow_forwardConyugated acid Conyugated bese Jamula ha Formula ha A NH 5.61010 CH,NH HCO 2.2 10-8 Complete the table below. B Sure cach OA Your cinowrentries has the Correct numbCr oA Signiticant digits. You may assime the tamparature is 25°C.arrow_forwardO Select the strongest acid. O I— ④SH HOH H OH Iarrow_forward
- B File Paste E + Home Cut E Copy 4 Page 1 of 1 Clipboard Format Painter Insert 0 words S Draw Design Layout References Calibri (Body) BIU U abe 11 À A Aa A A abe X₂ X² Font Type here to search P English (Philippines) Accessibility: Investigate Mailings 10 E•E•S• Review View Help HE e W Document1 - Word €¶ Paragraph 1-32. A bottle of concentrated aqueous sulfuric acid, labeled 98.0 wt% H₂SO4, has a concentration of 18.0 M. (a) How many milliliters of reagent should be diluted to 1.000 L to give 1.00 M H₂SO4? (b) Calculate the density of 98.0 wt% H₂SO4- S ■ Tell me what you want to do AaBbCcDc AaBbCcDc AaBbC AaBbCct AaB AaBbcct AaBbCcD Normal 1 No Spac... Heading 1 Subtitle Subtle Em... Heading 2 Title Styles Sign in 1:31 (Ctrl) - ENG S IX Share Find ab Replace Select Editing 8:09 pm 18/08/2022 ▸► + 228%arrow_forward11 of 11 I Review | Constants Periodic Table Suppose that you want to find the equation for a line that passes through the two points (0,3) and (4, 9). What is the slope of this line? Express your answer numerically. > View Available Hint(s) Πνα ΑΣΦ ? m = Submit rt C Complete previous part(s) 9:41 PM 36%arrow_forward1arrow_forward
- Complete each row of the table below by filling in the missing prefix. 1 1 Pa 1 1 Pa Pa = = Pa 10 10 = 10 10 6 -3 -6 3 Pa Pa Pa Pa H Xarrow_forwardPlease help me understand my HW question thanks!arrow_forward8. L0s t213.15= 318.18K 2. A woman is 5 ft 5 in tall and weighs 140 lb. Express these values in the SI system (m, kg)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY