
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
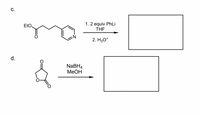
Transcribed Image Text:C.
1. 2 equiv PhLi
THE
EtO.
2. H30*
d.
NABH4
МеОН
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An aluminum cylinder is 0.512 ft in length and has a diameter of 0.3142 inch. The density of aluminum is 2.70 g / cc. MW of aluminum is 26.981538. What is the volume of the aluminum cylinder in cm3? What is the mass of the aluminum cylinder in g? Calculate the number of aluminum atoms present in the cylinder?arrow_forward+ e Home ourse.html?courseld=15539865&HepID=676f177415469ec3dc78ff3c8 bf724d9#1 0001 Inbox-Outlook We... S Sugarpill Cosmetic... Shop | Origami Owl Wholesale Glass M... S The Corinthian Hou... zed Invitat... HW Provide Feedback P Pearson Contact Us Copyright 2019 Pearson Education Inc. All rights reserved. Terms of Use Privacy Policy | Permissions MacBook Air »)) DD DII O00 F12 F11 F10 F9 F8 F7 F4 F6 F5 F3 + $ & 3 0 9 4 7 8 6 5 } { P E U R Y D L K G F H T 2LOarrow_forwardFinal temp?arrow_forward
- which ones are hete nogeneas mixture orange juice, soil, wine, air, vegetable soup. soil, sayp, or al Ok them? 2 or all Okthem?arrow_forward| ax NME /Chemical%20Reactions%20Asgn%202-5.pdf 9 Employee Home r4c Covenant Health Ca... Outlook.com - Free... S Birthday party politi. G what does it mea 1 / 2 100% Chemical Reactions-Write Neat and Balanced Reactions from Word Equations You need to submit one sheet of paper (not this one), that is very neat, with a fully balanced reaction for these 6 reactions Remember to include proper states. 1. solid potassium metal reacts with oxygen gas to produce solid potassium oxide. 2. solid potassium chlorate decomposes into oxygen gas and solid potassium chloride. 3. solid aluminum oxide is decomposed into solid aluminum and oxygen gas. 4. aqueous cobalt(III) nitrate reacts with solid zinc to produce aqueous zinc nitrate and solid cobalt.arrow_forwardI. г. H3CO 0 оснз ино нзот, нго ночно H2504arrow_forward
- ← → CO www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-JgXZp57itWHhRgilOD 0 Email M Gmail YouTube Maps MyCSU-Columbus... B Homepage - Georg... W Microsc O STATES OF MATTER Calculating mass percent composition V A chemist mixes 74.9 g of water with 80.1 g of 2-methylpyrazine and 34.8 g of acetic acid. Calculate the percent by mass of each component of this solution. Be sure each of your answer entries has the correct number of significant digits. component mass percent x10 water 0 % S ? 2-methylpyrazine 0% acetic acid 0% ||| esc Explanation Check Type here to search f2 ? f3 f4 X O i f5 16 a & O dip 17arrow_forwardtaprect number of significant figures. A. Precipitation of CaC,O4H,O from the Calculation Zone Salt Mixture mil o 3. Mass of salt mixture (g) Data Analysis, 1. 0.879 4. Mass of filter paper (g) 1.896 5. Mass of filter paper and CaC,O4•H2O (g) рaper 2.180 6. Mass of air-dried CaC,O4•H,O (g) B. Determination of Limiting Reactant Data Analysis, 3. 1. Limiting reactant in salt mixture CaCl, 2H,O 2. Excess reactant in salt mixture Data Analysis e cxbcipuu 1. Moles of CaC,O,'H,O precipitated (mol) Data Analysis, 4. Show calculation. 2. Moles of limiting reactant in salt mixture (mol) 20 See equation 8.1. 3. Mass of limiting reactant in salt mixture (g) Show calculation. trotanjl Data Analysis, 5. 4. Mass of excess reactant in salt mixture (g) Equals mass of salt mixture minus mass of limiting reactant. Show calculation. 5. Percent limiting reactant in salt mixture (%) Show calculation. 6. Percent excess reactant in salt mixture (%) rtc beccib For Trials 2 and 3, the percent CaCl,-2H,O in the…arrow_forwardP Pearson eText y! A sunscreen preparation co G ethyl alcohol, also known as Course Home ge.com Probienuz.108 - ENnanceO- WILN Feeuback I Review | Constan You may want to reference (Pages 42 - 45) Sections 2.6 and (Pages 46 - 50) 2.7 while completing this problem. Part A Ethyl alcohol, also known as ethanol, has a density of 0.79 g/mL. What is the volume, in quarts, of 3.25 kg of this alcohol? Express your answer using two significant figures. V = quarts Submit Previous Answers Request Answer X Incorrect; Try Again; 3 attempts remaining ovide Feedbackarrow_forward
- What is the molar mass of unknown Z, .0578g and 1.12 x10 -3moles. 2$ 4 5 dele R Y F G H. K Larrow_forwardFor each compound below, draw in all missing lone pairs and indicate whether each lone pair is delocalized or localized. Then, use that information to determine the hybridization state for each atom that has a lone pair. Hint: allylic lone pairs are delocalized and do not count towards the steric number.arrow_forward3. Convert 5.0 Tbytes to Mbytes. 5tutesx 0.000001 mby- ts - 5.00 0no mbytes 1 चि je %3D 1 fera 15.0x10 1. Subtract 3.58 x 102 kg from 1.41 x 101 ng and report your answer in g.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY