
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:A OnCourse Connect
> Assessment: Chemistry x
4 Copy of spread of isla x
E The Spread of Islam- G X
M Inbox (904) - aarojame
A Classes
b oncourseconnect.com/assessment/1651879/5287e2a3-0d0b-e2c0-c15c-3e68a37149b4
O TPSS Bookmarks
CHEMISTRY BENCHMARK TEST 01
CHEMISTRY I 12-7 (AARON JAMES, ID. 12390724)
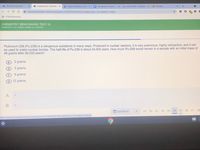
Plutonium-239 (Pu-239) is a dangerous substance in many ways. Produced in nuclear reactors, it is very poisonous, highly radioactive, and it can
be used to make nuclear bombs. The half-life of Pu-239 is about 24,000 years. How much Pu-239 would remain in a sample with an initial mass of
48 grams after 96,000 years?
2 grams
3 grams
6 grams
12 grams
A
Save/Exit
19 20 21 22 23 24 25 26 27 28
https://www.oncourseconnect.com/assessment/1651879/5287e2a3-0d0b-e2c0-c15c-3e68a37149b4#
國
O O O O
B.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Mg3(PO4)2 (s) Mg2*(aq) + _ PO43- (aq) Mg2* (aq) PO43 (aq) initial change eqm Write Ksn in terms of x. 36 x5 12 x3 3x4 108 x5arrow_forwardOnCourse Connect Assessment: Chemistry x Copy of spread_of_islar x = The Spread of Islam -G x M Inbox (904) - aarojame A Classes boncourseconnect.com/assessment/1651879/5287e2a3-0d0b-e2c0-c15c-3e68a37149b4 D TPSS Bookmarks CHEMISTRY BENCHMARK TEST 01 CHEMISTRY I 12-7 (AARON JAMES, ID: 12390724) A student dissolved 15 grams of pure acetic acid (CH,COOH) in enough water to make 100. milliliters of solution. Given the atomic masses provided on the periodic table, what is the molarity (M) of the acetic acid solution? a 0.025 molar D0.40 molar © 2.5 molar O 4 molar A B B 1 2 4 5 6 7 8 9 10 3. O Save/Exit 4 sn TESSarrow_forward/*arrow_forward
- 2. An 84 kg University of Toledo groundskeeper who is spraying a 100 mg/L solution of the insecticide dieldrin (MW = 381; logP = 4.56) on some trees exposes 100 cm“ of his skin to the solution over a 4 hr period. Dieldrin has a half-life of about 6 months, a volume of distribution of about 40 L/kg, and a dermal permeability of about 3 x 10° cm/sec. (a) Estimate the absorption rate during the exposure period assuming the blood concentration is much lower than the skin concentration (i.e., take the blood level to be zero in the skin absorption equation). (b) Estimate how it takes after exposure begins for the groundskeeper's plasma dieldrin levels to reach 1 ng/L. (c) Estimate the maximum plasma dieldrin concentration in ng/L that will occur in this groundskeeper due to this exposure. (d) Estimate how long it will take after exposure ends for the groundskeeper's plasma dieldrin levels to drop back down to 1 ng/L. %3Darrow_forwardChrome File Edit View History Bookmarks Profiles Window Help Tab 21% D BC Broward College | Affordable x O Onelogin 1A BC - Student Registration O Launch Meeting - Zoom ALEKS - Esther Octave - Le i www-awa.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IBcn9hvCfbYq_fi3Zsn8H2oW_5PTMi0acVC-XJc4zwaxK8Wf2BSBxnL8YsAgEaj1JZm27.. * E Apps M Gmail O YouTube Maps BC Broward College |. O Sample Source An... O New Tab G What does Duckw.. E Untitled documen. O CHEMICAL REACTIONS Calculating the heat of reaction from molar reaction enthalpy a... Esth A chemist measures the energy change AH during the following reaction: 2 Fe,O3(s) → 4 FeO(s)+O2(g) AH=560. kJ Use the information to answer the following questions. O endothermic. This reaction is... O exothermic. Suppose 27.6 g of Fe,O, react. O Yes, absorbed. O Yes, released. Will any heat be released or absorbed? O No. If you said heat will the second part of this question, calculate how much heat will be released or absorbed. released or absorbed…arrow_forwardQuestion 4arrow_forward
- Mla pnc.com/women Mail - Whi x O Grades for x Course Ho X G oxide cont X G Exercise 4.x E 4 - Molecu x O Bananas a X 1.84 x 10- x + envellum.ecollege.com/course.html?courseld 16985674&OpenVellumHMAC=a0b841caaee7feddab1623a6c83a92c0#10001 Review | Constants I Periodic Table Percent composition refers to the mass percent of each element in a compound: mass of element Part A mass percent = x 100% mass of compound > For example, the percent composition of water, H2O. is 11.2% hydrogen and 88.8% oxygen. Therefore, a 100-g sample of water contains 11.2 g of hydrogen atoms and 88.8 g of oxygen atoms. A hydrocarbon is a compound that contains mostly carbon and hydrogen. Calculate the percent composition (by mass) of the following hydrocarbon: CH22 Enter the percentages of carbon and hydrogen numerically to four significant figures, separated by commas. The periodic table will be useful when doing this problem. You can access a periodic table by clicking the "Tools" link in the upper right…arrow_forwardSolving part B and C E°cell from part A = 2.33Varrow_forwardWith the image attached please do the calculations/calculate what is highlighted in yellow please Please also calculate - percent % error in molar mass - percent % error in pKa Please please please answer as fast as possiblearrow_forward
- Fill in coefficientsarrow_forwardLIS 0 x Cee res te erE O Esoe 2011 O Empower 2021 A educate lindsay.k12.ca.us/iframe.aspx?Ctrl STUDENT BASE HOME CONTROL dsay Bookmarks A Classes O Google Sides O Empower 2020 E Google Docs t Lindsay High School R Phynics Safety & Sk E Guided practice 3L Flag for follow-up Group-12 )436 9 10 11 12 13 14 15 16 17 10 Irerlod The Perlodic Table of the Elements Elements in the same group tend to have similar characteristics, but some things like tendency to gain or lose electrons can still vary even within a group Which of the following is true regarding the elements in group 2? O A Be, Beryllium is the largest and has the greatest tendency to gain electrons. OB Be Beryllium and Ba, Barium will have the same tendancy to lose electrons OC Ra, Radium is the smallest and will have the greatest tendency to lose electrons. O D. Ba, Barium will have a greater tendency to lose electrons than Mg, Magnesium. DELL %23 E S A Carrow_forwardStudent Discipline Problems The data for a random sample of 32 months for the number of discipline problems that occurred each month at a large high school are shown. Find the 99% confidence interval for the mean when σ 3. Round your answers to one decimal place. = 13 19 10 11 14 25 16 27 22 29 23 29 28 17 17 22 28 26 29 20 22 18 14 215 16 27 17 14 17 110 11 20 19 22 Send data to Excel We are 99% confident that the mean is between 15.1 and 17.8 discipline problems per month.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY