
Chemistry In Focus
7th Edition
ISBN: 9781337399692
Author: Tro, Nivaldo J.
Publisher: Cengage Learning,
expand_more
expand_more
format_list_bulleted
Question
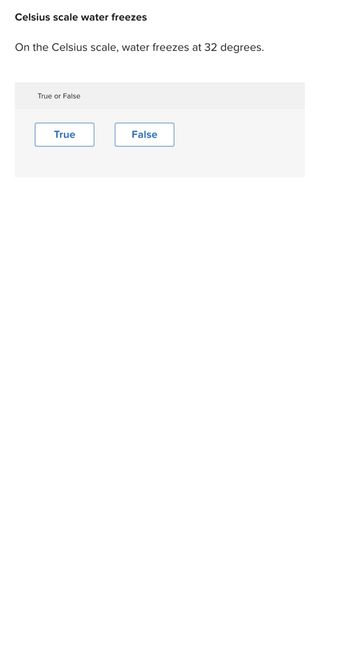
Transcribed Image Text:Celsius scale water freezes
On the Celsius scale, water freezes at 32 degrees.
True or False
True
False
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- solution is a homogeneous mixture. Can you give an example of a gaseous homogeneous mixture? A liquid homogeneous mixture? A solid homogeneous mixture?arrow_forwardAt what temperature does water freeze in kelvin? 100 K O 273 K O OKarrow_forwardWhat is happening to the water molecules at segment F? C A D El G B F Time Freezing, the water molecules are gaining energy to become a solid. Melting, the water molecules are gaining energy to become a liquid. Freezing, the molecules are losing energy to become a solid. Melting, the water molecules are losing energy to become a liquid. Temperaturearrow_forward
- X 26. In the question above, two liquids react together to form solid. What term is given to this process? Precipitation Effervescence O Clumping Freezingarrow_forwardBalance each chemical equation. NH2OH(g)→NH3(g)+N2(g)+H2O(g) Express your answer as a chemical equation. Identify all of the phases in your answer.arrow_forwardUse numbers to order the substances by increasing boiling point. Let #1 be the substance with the LOWEST boiling point and #3 be the substance with the HIGHEST boiling point. Hydrogen Sulfide, H2S Methane, CH4 Carbon Dioxide, CO2 o=c=ö H.arrow_forward
- 5. Calculate the amount of energy given off when 13g of water at 51° C freezes to ice at 0°C. Did a phase change occur? If so, which one? Is this endothermic or exothermic?arrow_forwardWhich is NOT directly attributable to a property of water? O The top of a lake freezing C Nutrients can dissolve and chemical reactions can occur O A small insect's ability to walk on water O The low boiling point of waterarrow_forwardAnswer questions 4-5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning