
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
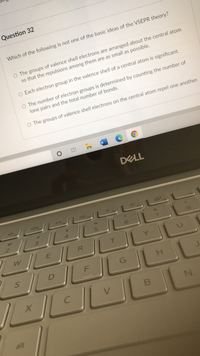
Transcribed Image Text:Question 32
Which of the following is not one of the basic ideas of the VSEPR theory?
O The groups of valence shell electrons are arranged about the central atom
so that the repulsions among them are as small as possible.
O Each electron group in the valence shell of a central atom is significant.
O The number of electron groups is determined by counting the number of
lone pairs and the total number of bonds.
O The groups of valence shell electrons on the central atom repel one another.
DELL
R
F
V.
alt
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- For each row in the table below, decide whether the pair of elements will form a molecular or ionic compound. If they will, then enter the chemical formula of the compound. If the elements will form more than one compound, enter the compound with the fewest total number of atoms. You may assume all chemical bonds are single bonds, not double or triple bonds. element #1 element #2 compound formed? chemical formula ionic iodine cesium O molecular ? neither jonic hydrogen охудen O molecular O neither O ionic calcium sodium O molecular neitherarrow_forwardFor each row in the table below, decide whether the pair of elements will form a molecular or ionic compound. If they willI, then enter the chemical formula of the compound. If the elements will form more than one compound, enter the compound with the fewest total number of atoms. You may assume all chemical bonds are single bonds, not double or triple bonds. element #1 element #2 compound formed? chemical formula jonic barium bromine O molecular O neither O ionic hydrogen oxygen O molecular O neither O ionic rubidium iodine O molecular neither MAR X P. 27 MacBook Air DD 000 80 11 F9 F10 F7 FB F4 F5 F6 F2 F3 @ $ & 3 4 6 8 9 * COarrow_forwardWhich of the following is an example of a compound having both ionic and covalent bonds? O sodium nitrate O magnesium carbonate O calcium hydroxide O all of the above 目 DELLarrow_forward
- 13. Complete the following table with the number of electrons and electron pairs shared in different types of covalent bonds. Also give an example of diatomic element that exhibits this type of bonding Covalent Bond Single Double Triple Electron pairs Electrons Example (Chemical Formula)arrow_forwardWhich of the following statements is true for an ionic bond? in the picture.arrow_forwardI'm having some trouble on my homework and could really use some help See attached:arrow_forward
- For each row in the table below, decide whether the pair of elements will form a molecular or ionic compound. If they will, then enter the chemical formula of the compound. If the elements will form more than one compound, enter the compound with the fewest total number of atoms. You may assume all chemical bonds are single bonds, not double or triple bonds. element #1 barium argon carbon element #2 compound formed? chemical formula O ionic O molecular O neither O ionic sulfur helium iodine O ο οίο ο ο molecular O neither O ionic O molecular Oneither 0 0 X Sarrow_forwardWhich phrase best defines a covalent bond? Ostrong polar attraction o electrons transferred between atoms O weak polar attraction O electrons shared between atoms andarrow_forwardThe molecule PH3 has… define total electrons, shape, molecule contains, triple bonds, and wether it is polar or non polar.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY