
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Transcribed Image Text:CHEM 1300 E01/E02
<Post Lecture Homework Chapter 04
Exercise 4.106
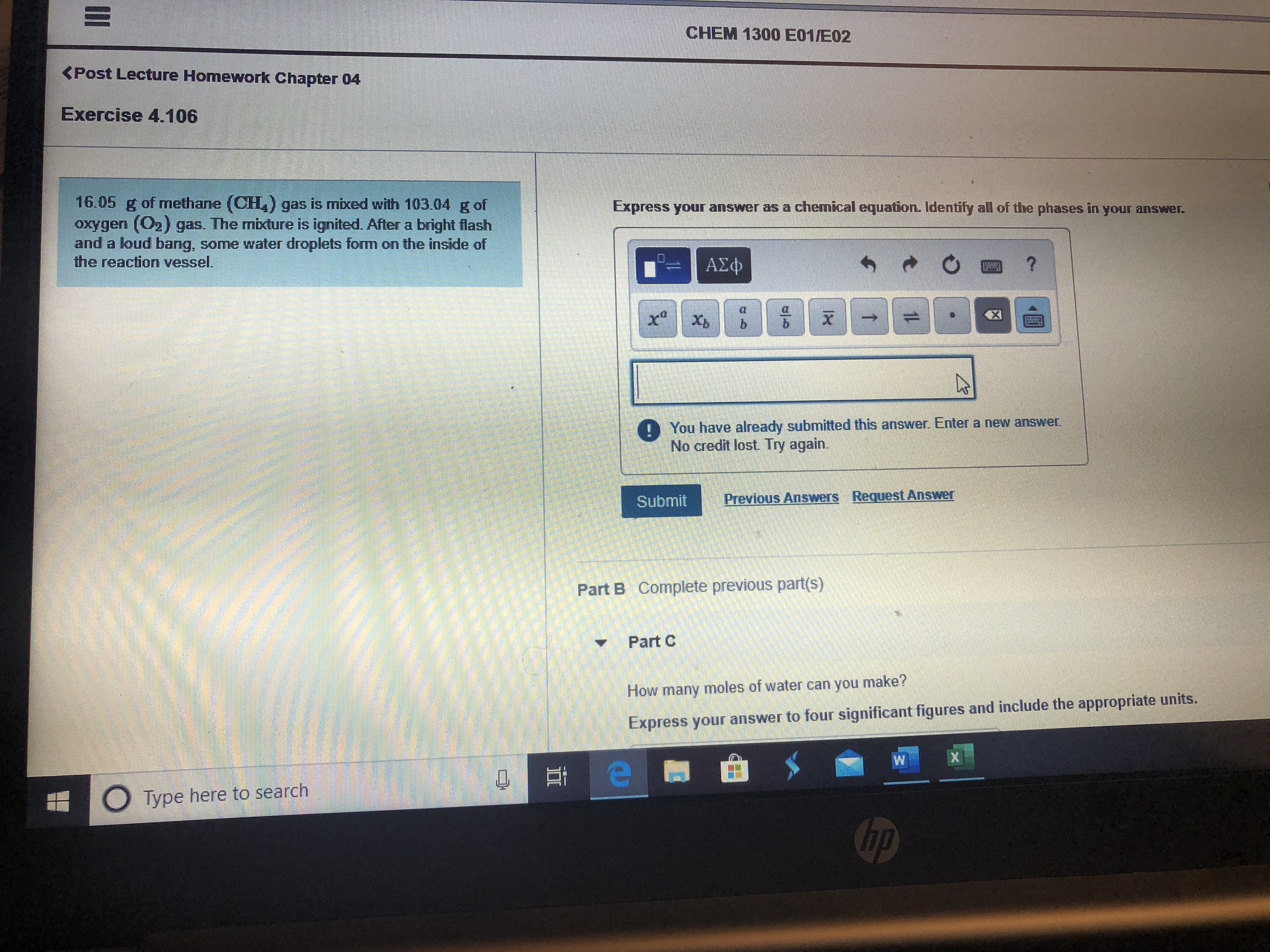
16.05 g of methane (CH,) gas is mixed with 103.04 g of
oxygen (O2) gas. The mixture is ignited. After a bright flash
and a loud bang, some water droplets form on the inside of
the reaction vessel.
Express your answer as a chemical equation. Identify all of the phases in your answer.
ΑΣφ
x Xp
b.
You have already submitted this answer. Enter a new answer.
No credit lost. Try again.
Submit
Previous Answers Request Answer
Part B Complete previous part(s)
Part C
How y moles of water can you make?
many
Express your answer to four significant figures and include the appropriate units.
O Type here to search
hp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- drc%3D0&gi=24758088ctgl=18dnb 06 What is the molarity of a solution prepared by dissolving 213.6 grams of Fe(OH)3 in enough water to produce a total volume of 1.5 L? Round your answer to 1 decimal place. Your Answer: Answer units D Add attachments to support your workarrow_forwardPlease help with question 3arrow_forwardWine goes bad soon after opening because the ethanol (CH3CH₂OH) in it reacts with oxygen gas (0₂) from the air to form water (H₂O) and acetic acid (CH₂COOH), the main ingredient of vinegar. What mass of water is produced by the reaction of 8.9 g of ethanol? Round your answer to 2 significant digits. 08 g x10 ? Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility Explanation Check X MacBook Pro & Xarrow_forward
- Ex. 110 - Calculating Mass of 3 attempts left Check my work What mass of oxygen is needed to react with 1.79 gal of methanol according to the balanced equation below? (1.00 gal = 3.79 L, and the density of methanol is 0.793 g/mL.) 2 CH3OH() + 3 02g) – 2 CO2g) + 4 H2O(g) If appropriate, express your answer in scientific notion. (Click on the answer box to show the pallet.) < Prev 3 of 5 ***** ****arrow_forward27 A chemist prepares a solution of sodium chloride (NaCl) by measuring out 0.50 g of NaCl into a 250. mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Cl anions in the chemist's solution. do Be sure your answer is rounded to 2 significant digits. 18 Ar mol x10 L Submit Assignment Continue 2022 McGraw Hill LLC. AIl Rights Reserved. Terms of Use Privacy Center Accessibility Show All IMG-6095.jpg IMG-6096.jpg IMG-6097.jpg IMG-6098.jpg IMG-6099.jpg MacBook Air DD DII F12 F11 F10 F9 80 F7 F8 F6 F5 F4 esc F2 F3 F1 & % @ # 7 8 3 4 5 6 1 2 { P E R Y Q W %24arrow_forward62. Antacid Fizz When an antacid tablet dissolves in water, the fizz is due to a reaction between sodium hydrogen carbonate (NaHCO3), also called sodium bicarbonate, and citric acid (H₂CH₂O₂). 3NaHCO3(aq) + H₂CH₂O,(aq) → 3CO₂(g) + 3H₂O(l) + Na, C₂H₂O₂(aq) How many moles of Na₂C,H,O, can be produced if one tablet containing 0.0119 mol of NaHCO is dissolved?arrow_forward
- Complete the following questions using 6 iron (Fe) atoms for each request. a. Draw, on the particulate level, the solid, liquid, and gas phases of 6 Iron (Fe) atoms in each phase. a1.10 bu aid E b. Write the chemical equation (symbolic representation) of iron changing from a gas phase to a liquid phase. c. Describe what you would observe while watching iron change from a liquid phase to a solid phase.arrow_forwardRemaining Time! 02:29:4. Consider the balanced chemical equations shown below. When a 6.00 g sample of a mixture of iron (Fe) and aluminum (Al) is treated with excess HCl(aq), 0.177 moles of H2 are obtained. What is the mass percentage of Fe in the original mixture? Choose the closest answer. Fe(s) + 2 HCI(aq) FeCl2(aq) - + H2(g) 2 Al(s) +6 HC((aq) 2 AICI3(aq) + 3 H2(g) 96 % 69 % 47 % 33 % 17 % Rack Question Menu - O Oarrow_forwardHow can we find the percentage? And the produced?arrow_forward
- Z @ ▶ 12. ✿ Text-to-Speech You are asked to make 43.0 grams of iron (Fe) from iron III oxide (Fe:O) and carbon monoxide (CO) as shown in the chemical equation below. Fe₂O3 + 3C0→2Fe + 3C0₂ How many grams of iron (III) oxide must you use? OA. 61.5g OB. 122 g OC. 0.777 g OD. 686 g 1246 2 W S # X 15 # 3 E 14 H L $ 4 Q Search R D F % 5 T CV 6 b VI B | & Y lipi GH 7 Lo *K U N 8 J 19 144 9 K M fo Dl O In DDI P : [ = ? 1 F 10 10 10 10 1arrow_forwarddecomposition: HgO(s) → Express your answer as a complete chemical equation, with reactants and products. the phases in your answer.arrow_forwardSample 1 Sample 2 Sample 3 Mass of Erlenmeyer Flask (g) 24.33 24.37 24.44 Mass of Erlenmeyer Flask + Calcium Hydroxide Solution (g) (g)(lime water) 27.22 27.29 27.33 Mass of Calcium Hydroxide Solution (g) 2.89 2.92 2.89 Volume of Ca(OH)2 Density = 1.000 g/mL 2.89 2.92 2.89 Concentration of HCl (M) 0.1 0.1 0.1 Initial HCl Volume in Syringe (mL) 1.00+1.00 1.00+1.00 1.00+1.00 Final HCl Volume in Syringe (mL) .72 .66 .54 Volume of HCl Delivered (mL) 1.28 1.34 1.46 Moles of HCl Delivered Moles of OH- in Sample Moles of Ca2+ in Sample Molar Solubility (M) Calculated Ksp Average Calculated Ksp I am very confused how to solve the blank spots in the table. Everything including and after "Moles of HCl Delivered" confuses me. Please help me figure out how to solve the sections correctly. Also feel free to…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY