
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
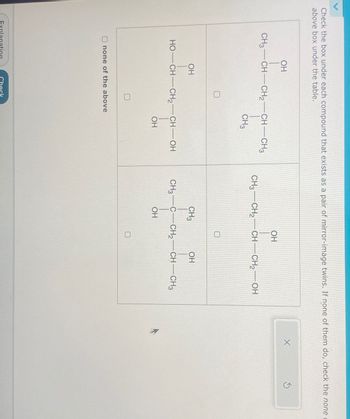
Transcribed Image Text:Check the box under each compound that exists as a pair of mirror-image twins. If none of them do, check the none
above box under the table.
OH
CH3-CH-CH2-CH-CH3
OH
CH3 CH2 CH CH2OH
CH3
0
OH
HO—CH—CH,—CH—OH
OH
CH3
OH
CH3 C-CH2-CH-CH3
OH
none of the above
Explanation
Check
O
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Determine whether each of the following molecules is a hemiacetal, acetal, or neither and select the appropriate box in the table. O hemlacetal O acetal O neither CH₂ CH-O-CH2-CH3 Ohemiacetal O acetal CH₂-0-C-O-CH2-CH2-CH3 O neither CH3 CH3 CH₂ CH-OH CH3 CHOCO–CH €€ CH₂ CH2 CH3 CH₂ Ohemiacetal acetal O neither HO O HO OH Ohemiacetal O acetal O neither OH x امیarrow_forwardH3C-N 'N CH3 CH3 N N lol H3C Hofer CH3 N,N-Dimethyloxamidearrow_forwardWrite the complete common (not IUPAC) name of each molecule below. Note: if a molecule is one of a pair of enantiomers, be sure you start its name with D- or L- so we know which enantiomer it is. molecule i H3N + NH3 common name (not the IUPAC name) H N NH ☐ شهدة + NH3 | ☑arrow_forward
- Select the appropriate name for each compound. gr 2-phenylpentyl methanoate methyl 2-phenylhexanoate 5-phenylhexyl methanoate methyl 5-phenylhexanoate CH3CH₂CHCH₂CO2(CH2)3CHCH3 L CH₂CH3 2-ethylbutyl 4-chloropentanoate 4-chloropentyl 3-ethylpentanoate 4-chloropentyl 2-ethylbutanoate 3-ethylbutyl 4-chloropentanoate CH3CH2CO2(CH2)3CH3 butyl propanoate ethyl butanoate butyl ethanoate propyl butanoatearrow_forwardGive the proper IUPAC name for the molecule shown as a Fischer projection. Make sure to include the correct (R) or (S) designation where appropriate. CH₂CH3 Br H CH₂CH₂CH3 The name of the molecule is: O (R)-4-bromohexane (S)-3-bromohexane (R)-3-bromohexane (S)-4-bromohexanearrow_forwardPlease help with row 3: Follow the instructions in each column. Hint for the last column: draw it with a wedge and again with a dash – which gives the correct configuration?arrow_forward
- Please help with row 2/question 2 ; the instructions for the specific column in row 2 are above row 1: Follow the instructions in each column. Hint for the last column: draw it with a wedge and again with adash – which gives the correct configuration?arrow_forwardi need help with both pleasearrow_forwardPlease help with row 1, highlighted:arrow_forward
- Answer the following questions based on these structures. Here is the terminology you can use to describe the relationships. Structural isomers, Enantiomers, Diastereomers, and no relationship. CHO CHO -Н H- I CHO HO-H H- -OH -OH CH₂OH 1 I НО- OH I I CHO -H H -ОН -ОН HO I CH₂OH =0 H -OH CH₂OH 2 H H но Но CHO НО H H НО -ОН -ОН -H -H CH2OH 8 -H OH OH H CH₂OH 3 I НО CH₂OH 7 What is the relationship between 1 and 4? What is the relationship between 1 and 3? What is the relationship between 1 and 5? What is the relationship between 2 and 9? What is the relationship between 4 and 10? What is the relationship between 5 and 6? What is the relationship between 2 and 10? CH2OH -ОН -Н CH2OH 9 НО H- H CH2OH 0 H -ОН ОН CH2OH Н- НО- НО- НО НО H- Н- CH2OH -0 -ОН -H -H CH2OH 10 H -OH ОН CH2OH 5 Н НО НО- H- CHO OH -Н H OH CH₂OH 6arrow_forwardFor each pair of compounds, please explain if they are identical. comstitutional isomers, enantiomers, or diastereomers.arrow_forwardWhich compound is a meso isomer? Br H-CH₂ H₂C H H -Br CH3 A Br -H H- -Br H- CH₂ B CH₂ -Br H -Br H₂C CH3 с Br Br D I -CH₂ Br- -CH₂ -H H₂C- Br hill.com:80/olcweb/cgi/pluginpop.cgi? E onClick="window.open('http://highered.mcgraw- (29.0K)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
