
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Draw a structural formula for the major organic product of each reaction and specify the most likely mechanism by which each is formed.
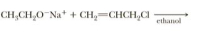
Transcribed Image Text:CH;CH,O¯N + + CH,=CHCH,CI
ethanol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 8) Show how the following conversions might be accomplished, including all reagents and intermediates. Br Y. OHarrow_forwardOct-1-yne (HC≡CCH2CH2CH2CH2CH2CH3) reacts rapidty with NaH, forming a gas that bubbles out of the reaction mixture, as one product. Oct-1-yne also reacts rapidty with CH3MgBr, and a different gas is produced. Write balanced equations for both reactions and identify the gases formed.arrow_forwardWhich is the major isolated organic product of this reaction? A) + D) Pd(PPH3)4 NaCO3arrow_forward
- Show the arrow pushing mechanism for the following reactionarrow_forwardProvide a detailed, stepwise mechanism for the following reaction. Br NaNH, NH2 + NaBrarrow_forwardAlkyl halides undergo nucleophilic substitution and elimination reactions. When the kinetics of the reaction are measured, if the rate of the reaction is found to be dependent only upon the concentration of the alkyl halide the reaction is first order. The substitution reaction is thus termed SN1, and the elimination reaction is termed E1. These reactions are unimolecular and occur in two steps. The first step is rate-limiting and involves the loss of the leaving group to form a carbocation. In the second, fast, step the nucleophile adds to the carbocation in the SN1 reaction or elimination occurs to give an alkene in the E1 reaction. Because the carbocation is planar, the nucleophile can add to either face and therefore racemization is usually observed although solvent effects can influence this somewhat. E1 elimination follows Zaitsev’s rule and typically yields the most substituted alkene as the major product. Conditions which favor the SN1/E1 pathway include the use of a weak…arrow_forward
- the organic compound 2-heptanone, belonging to the ketone family, is responsible for the strong penetrating odor in Roquefort cheeses. Starting from acetylene as the starting reagent, propose a synthesis line with the reaction mechanisms involved for the synthetic obtaining of 2-heptanone and use it as a food additive in analogous cheeses.arrow_forwardSuggest a step by step mechanism for this reaction.arrow_forwardJj.153.arrow_forward
- What is the hybridization and electron geometry of the N, C and interior O in CH3NO2 (lone pairs not shown)arrow_forwardProvide the final product and mechanism for the following reactionsarrow_forwardPredict the coupling products of organometallic substitutions, and use them in syntheses.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY