
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
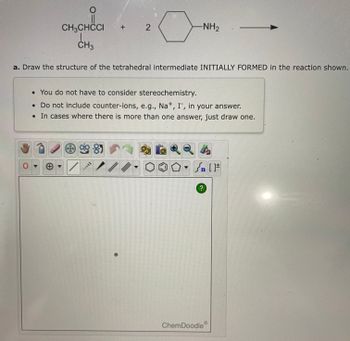
Transcribed Image Text:CH3CHCCI
CH3
O
+
a. Draw the structure of the tetrahedral intermediate INITIALLY FORMED in the reaction shown.
*
2
9985
-NH₂
• You do not have to consider stereochemistry.
• Do not include counter-ions, e.g., Na+, I, in your answer.
. In cases where there is more than one answer, just draw one.
?
ChemDoodle
LET
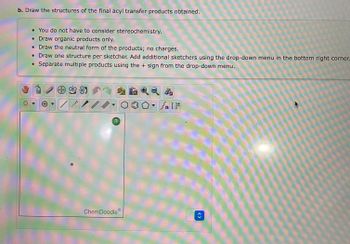
Transcribed Image Text:b. Draw the structures of the final acyl transfer products obtained.
• You do not have to consider stereochemistry.
• Draw organic products only.
• Draw the neutral form of the products; no charges.
• Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner.
• Separate multiple products using the + sign from the drop-down menu.
1
***
?
ChemDoodle
Sn [F
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- -OH a. Draw the structure of the tetrahedral intermediate INITIALLY FORMED in the reaction shown. • You do not have to consider stereochemistry. • Do not include counter-ions, e.g., Na*, I¯, in your answer. • In cases where there is more than one answer, just draw one. - √n [F ?arrow_forwardDraw a structural formula for the intermediate in the following reaction: CH₂Cl₂ Br₂ • You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. • Do not include counter-ions, e.g., Na*, I, in your answer. *** ▼n [F ChemDoodleⓇ laarrow_forwardDraw a structural formula for the intermediate in the following reaction: C ▼ + 11... Cl₂ . You do not have to consider stereochemistry. . You do not have to explicitly draw H atoms. • Do not include counter-ions, e.g., Na*, I, in your answer. C P opy CH₂Cl₂ aste []* ?arrow_forward
- CH3CH2CH2CH2CH2CH2CI 2 NH3 a. Draw the structure of the tetrahedral intermediate INITIALLY FORMED in the reaction shown. • You do not have to consider stereochemistry. • Do not include counter-ions, e.g., Na", I', in your answer. • In cases where there is more than one answer, just draw one. C opy aste (Previous Next ChemDoodle Email Instructor Save and Exitarrow_forwardNonearrow_forwardPlease do both Part A and B and make answers clear!! thank you!arrow_forward
- Draw a structural formula for the intermediate in the following reaction: Br2 1... CH₂Cl₂ You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. • Do not include counter-ions, e.g., Na*, I, in your answer. ChemDoodleⓇarrow_forwardDraw a structural formula for the more stable carbocation intermediate formed H3C C=CH2 + HBr H3C • You do not have to consider stereochemistry. • Do not include anionic counter-ions, e.g., I, in your answer. • For cases in which carbocations of the same or similar stability are expe structures. ⚫ Draw one structure per sketcher. Add additional sketchers using the dr right corner. ⚫ Separate structures with + signs from the drop-down menu. ? √n [arrow_forwardPlease don't provide handwritten solution .....arrow_forward
- Please don't provide handwriting solutionarrow_forwarded Draw a structural formula for the more stable carbocation intermediate formed in the reaction shown. CH3 . H₂C 0- H₂C • You do not have to consider stereochemistry. • Do not include anionic counter-ions, e.g., I, in your answer. • For cases in which carbocations of the same or similar stability are expected, draw all of the structures. ▾ • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down menu. + HBr C(CH3)3 *** Y [ ] درarrow_forwardDraw the structure(s) of the major organic product(s) of the following reaction. 1. Dry Et₂O + CH3Mgl H 2. aqueous HCI at 0° • You do not have to consider stereochemistry. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. + √n [ ? ChemDoodlearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY