
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
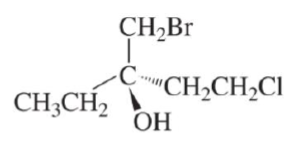
Transcribed Image Text:CH3CH2
CH₂Br
"CH₂CH₂CI
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 1 steps

Knowledge Booster
Similar questions
- Perform the conversions between energy units. 633 kJ = 2325 kcal %3D kJ 9.01 x 10 J = kcalarrow_forwardNonearrow_forward3) How many liters of combusted propane (C3H8; 44 g/mol) are required to heat a 100 kg steel pipe (specific heat of steel is 0.420 J/g°C), from room temperature (20°C) to its melting point of 1300°C? The density of propane is 0.493 g/mL.C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(l) ΔH=–2220 kJ /mol 4) How much energy is required to produce 1.9 grams of glucose (C6H12O6; 180 g/mol) from CO2 and H2O. Use the following combustion equation to help you answer the question?6CO2(g) + 6H2O(l) → C6H12O6(s) + 6O2(g) ΔH= 2900kJ 19) What volume of 0.12 M Na2SO4 will be required to react with 35 mL of 0.05 M CrCl6? 24) In an experiment when 100 g of C3H8 is combusted, 30 g of carbon dioxide resulted. What was the percent yield for the reaction?arrow_forward
- A calorimeter is calibrated to have a heat capacity of 441.2 J/°c. 0.143 g of substance X is burned in the calorimeter and raises its temperature from 23.1 °c to 39.5°c. What is the heat of combustion of substance X in J/g?arrow_forwardIf a "classic" SUV features a 52 gallon (197 L) gas tank and assuming complete combustion and a molecular formula of C3H18 for gasoline, how many L of air are consumed per tank of gasoline. Recall that air is 21% oxygen and assume a density of 0.78 g/mL for gasoline.arrow_forwardWhen 3.02 g of NHẠCI is dissolved in enough water to make 20.05 mL of solution, the temperature dropped from 19.8°C to 9.1°C. Calculate the enthalpy change (in kJ) when 1 mole of NH4CI is dissolved in water. The density and specific heat of water are 1.00 g/mL and 4.18 J/g°C respectively. Type your numeric answer and submitarrow_forward
- 50 g of solid sodium hydroxide is dissolved in 175 mL of water. Using a coffee-cup calorimeter, the temperature change of the water is measured to be -2.1°C. Which equation best describes this system? NaOH(s)→NaOH(aq) + kJ NaOH(s)+ kJ →NaOH(aq) NaOH(s)→NaOH(l) + kJ NaOH(aq)→NaOH(s) + kJ NaOH(aq)+ kJ →NaOH(s) NaOH(l)+ kJ → NaOH(s) What is the definition of the temperature of a substance? the speed of the slowest particles in the substance, subtracted from the speed of the fastest particles. the heat capacity of the substance times its mass a measure of the average kinetic energy of a system the total heat content of a substance the speed of the fastest particles in the substance Which statement describes an endothermic reaction? The surroundings warm up. The system releases energy. The potential energy of the products is less…arrow_forwardWrite a balanced equation for the complete combustion of propane, C3H8. Phases are optional.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY