
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
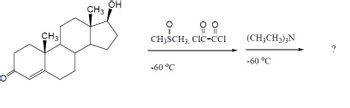
Transcribed Image Text:CH3
CH3
OH
요요
CH3SCH3 CIC-CC1
-60 °C
(CH3CH₂)3N
-60 °C
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2.) What is the heat energy needed to raise the temperature of 6.63moles of ethanol CH3CH2OH from a temperature of 2.33°C to 17.5°C. [CH3CH2OH=46.07g/mol] [CCH3CH2OH=2.46J/g°C]arrow_forwardFor each pair of compounds listed, check the box next to the one with the higher boiling point. compounds higher boiling point Si,H6 Si,H8 CH,CH, CH,CH,CH, Kr Nearrow_forwardCan you please answer these three sub problemsarrow_forward
- 7. Which of the following compounds has the highest boiling point? (1) Н-0 (2) H2S (3)H2Se (4)H,Tearrow_forwardChoose the compound with the HIGHEST BOILING POINT. -CH2 H₂c- NH₂ CH₂-C CH₂ о молниеносно то H,C CH OH _CH_ CH₂ CH₂ HC-CH2 CH₂ CH₂ 0 CH₂-CH₂ CH₂ OHarrow_forwardThe boiling point of water is about 200°C higher than one would predict from the boiling points of hydrogen sulfide and hydrogen selenide. One may explain this apparent anomaly by which of the following? A. The H-O covalent bond is much stronger than the H-S and H-Se bonds B. Water has the lowest molecular weight C. The intermolecular attractive forces are much greater in water than in hydrogen sulfide and hydrogen selenide. D. Water is less polar than hydrogen sulfide and hydrogen selenide. O A B C ODarrow_forward
- Convert Y8mminto dm Cuse 3 coersion factoarrow_forwardNEITEHR OF THE ANSWERS SHOULD BE PSI -60 PLEASE IF YOU PUT THAT I WILL DISLIKE AND If you copy ur answers from other bartleby answers i will dislikearrow_forwardRank the following compounds according to increasing solubility in gasoline. (1) C6H5CH3; (II) C6H5CI; (III) C6H5OH; (IV) C6H5SH (A) III < IV < I| < | (B) I < || < IV < III (C) I < || < III < IV (D) IV < III < I < ||arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY