
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
When a question asks about hydrogen bonding, is it referring to hydrogen bonding with the same molecule? For example (see picture attached), even though the first compound can form hydrogen bonds with alcohols, the answer key says that it cannot form hydrogen bonds.
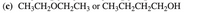
Transcribed Image Text:(c) CH;CH,OCH,CH3 or CH3CH2CH,CH2OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following best exhibits hydrogen bonding? Explain your answer. H2Se CH4 HBr NH3arrow_forwardPlease help me complete this questionarrow_forwardWhich of the following molecules display hydrogen bonding? Select ALL that display hydrogen bonding. a. CH3CH2OCH3 b. H2O c. O2 d. CH3COCH3 e. HOCH2CH3arrow_forward
- Label each molecule with the strongest intermolecular force exhibited in the pure substane.arrow_forward1.) Are there any IMF’s between cyclohexane molecules and vegetable oil? What kind(s)? Given this, what would the magnitude and sign of DHMIXING be for cyclohexane dissolving in vegetable oil? a) Are there any IMF’s forces between cyclohexane molecules and other cyclohexane molecules? What kind(s)? Given this, what would the magnitude and sign of DHSOLUTE be for cyclohexane dissolving in water? b) Are there any intermolecular forces between vegetable oil molecules and other vegetable oil molecules? What kind(s)? Given this, what would the magnitude and sign of DHSOLVENT be for cyclohexane dissolving in vegetable oil? c) Why do you think cyclohexane dissolves in vegetable oil? Analyze this the way it was done in class, thinking about the three contributions to DH What do you think the magnitude and sign of DHSOLUTION would be for dissolving cyclohexane in veg. oil? (Explain.) Would you expect these substances to dissolve in each other? Why?arrow_forward38)arrow_forward
- Hydrogen bonding with water Draw the expanded structure of C15H11I4NO4 (Levothyroxine) molecule. Illustrate all ways that this molecule could form hydrogen bonds with water. Do this by drawing bent water molecules as necessary and use dashed lines (---) to show H-bonding between water and the appropriate atom in the molecule. Be sure that it is very clear which atoms on each molecule are involved in the hydrogen bonds. Keep in mind that hydrogen bonds from water can only from to the polar parts of this molecule. If this molecule is not capable of forming hydrogen bonds with water, fully explain why not. Thank you!arrow_forwardBased on your knowledge of boiling point trends in alkanes, predict which compound has the higher boiling point, butane or 2-methylpropane (isobutane). Based on your knowledge of boiling point trends in alkanes, predict which compound has the higher boiling point, butane or 2-methylpropane (isobutane). a. isobutane would have a higher boiling point, since the greater the surface area, the higher the boiling point. b. Butane would have a higher boiling point, since the greater the surface area, the higher the boiling point. c. Butane would have a higher boiling point, since the smaller the surface area, the higher the boiling point. d. Isobutane would have a higher boiling point, since the smaller the surface area, the higher the boiling point.arrow_forwardArrange the following molecules in increasing order of boiling points. OH A В C D E Farrow_forward
- Rank the following in terms of increasing boiling point. (use 4 for the lowest boiling point and 1 for the highest boiling point) он O. он ноarrow_forwardWhat kind of intermolecular forces act between a chloromethane (CH3C1) molecule and a hydrogen sulfide molecule? Note: If there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.arrow_forwardWhich of the following intermolecular forces hold ethanol together? ion-dipole forces H-bonding, dispersion, and dipole-dipole forces dispersion forces H-bonding onlyarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY