
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Conditions to get to these products?
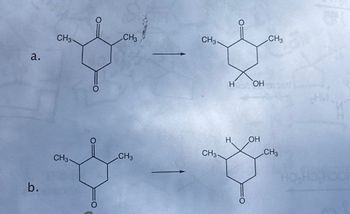
Transcribed Image Text:b.
CH3
CH3-
CH3
CH3-
a.
H
OH
«НИ
H
OH
CH3-
CH3
CH3
CH3-
O:
Ho Ho Hoo
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 10. Which of the below compounds are protic solvents? II II Al N-d-N HO-O H OH HO N. H3C H. Ethanenitrile (Acetonitrile) Ethane-1,2-diol (Ethylene glycol) Hexamethylphosphorotriamide Methanamide (Formamide) Methanol (HMPA) All are protic b. 1,1l and V are protic Il and IIII are protic a. d. 1, IV and V are protic е. All are aprotic C.arrow_forward3. Standard white vinegar you can buy in the grocery store is 5% concentration. That means 5% of the liquid vinegar is acetic acid and 95% of the solutioni is water. In a hardware store, you can buy industrial strength vinegar, which is 30% concentration. This means that 30% of the vinegar is acetic acid, and the remaining 70% is water. Samuel does another experiment, this time with 5% vinegar and 30% vinegar. He sets up two science fair volcanoes (in no particular order), each with the same temperature, mass of baking soda and volume of vinegar. But one volcano uses 5% vinegar and the other volcano uses 30% vinegar. He measures the volume of gas production for the'first minute of each reaction, and he records the data below. Volcano # 1 Volume of gas produced (m) vs Time (s) for Volcano t Time (s) Volume of gas produced (mL) 10 25 38 40 20 30 46 30 Volume of gas produced (ml) 50 40 50 52 60 53 40 Time ( 20 60 Volcano 2 Volume of gas produced iml) vs. Time is) for Vocano 2 Volume of…arrow_forwardhow would you do 4c? this is a non graded practice worksheetarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY