
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
"Give IUPAC for the following
I am absolutely stuck
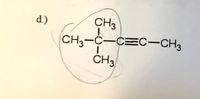
Transcribed Image Text:d.)
CH3
CH3-C-C=C-CH3
ČH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the iupac name of the moleculearrow_forwardNorethynodrel, a component of the first combined oral contraceptive, has the following structure. Identify the functional groups indicated in Norethynodrel. II IV OH O 1 = aldehyde; II = alkyne; I|| = alcohol; IV = alkene O1 = ketone; || = alkene; III = alcohol; IV = alkyne O 1 = ketone; || = aromatic; I|| = alcohol; IV = alkyne O 1 = anhydride; II = alkene; III = carboxylic acid; IV = alkene O 1 = ester; II = alkene; III = alcohol; IV = alkynearrow_forward1 M B W Chapter... C G Provide the systematic (IUPAC) name for the following hydrocarbons. (a) (b) H3C- Marvin JS H₂ P1₂₂ HC H₂C H₂ H₂C-CH₂ H₂ H₂ CH3 H₂ Write the structural formulas for the following hydrocarbons. (c) 2,2-dimethylbutane G ↑ webassign.net Help C MacBook Airarrow_forward
- "Give IUPAC for the following alkanes, alkenes, and alkynes" I am absolutely stuckarrow_forward09:49 < Back CHE_3100_WS3.1.pdf Organic Chemistry I Dashboard a) C6H10 b. Skeletal Isomers: Isomers that result from the reorganization of the carbon backbone are called structural isomers. Usually, these are easy to spot using IUPAC nomenclature; if the name of the parent compound of one molecule is different from the other molecule that it is being compared to, then the carbon skeleton has been reorganized and thus they are skeletal isomers of each other. WS-3.2: Draw skeletal isomers for the following molecules and provide an IUPAC name for each structure. Two isomers per compound are sufficient for this exercise. b) C4H8 c) CsH100 Dk d) بلوه Organic Chemistry I B goo Calendar 2 Shailesh Ambre To Do c. Positional Isomers: Positional isomers have the same parent compound (root name), but the position of the substituents differ. To identify molecules that share a positional isomer relationship- i. First, check if the root name is the same. ii. Second, check all substituents and…arrow_forwardN|ON|OU|G fic app.101edu.co Maps YouTube 1 N L H3C-CH₂ H3C Q A N G A © 2 - F2 W S C= X H Gb| CCC |2bA|QQ Question 7 of 31 The molecule shown here is classified as what type of organic compound? A) alkane CH3 B) alkene C) alkyne CH₂-CH3 D) aromatic compound E) aldehyde V Aa v > #3 II > 80 F3 E D C $ 4 Q F4 R F D, 67 dº % 5 V F5 T BAX G d G MacBook Air F6 B Y & 7 H F7 U N * 8 J DII F8 M ( 9 K S|G1|CS UONEE F9 O ) H L 7 F10 Done P F11 B ← L x Gra + 11 KR ☐ F12 +arrow_forward
- = O HYDROCARBONS Naming unbranched alkenes and alkynes Name these organic compounds: Explanation structure CH₂ C CH CH CH CH=CH=CH – CH Check X name 0 0 3 MacBook Pro D 3/5 Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terms of Use Privarrow_forwardAn alkane has 70 hydrogen atoms. How many carbon atoms would it contain if it were (a) a straight-chain alkane; (b) a branched-chain alkane; (c) a cycloalkane? (a) C's (b) C's (c) C'sarrow_forwardRank the following substituents in increasing bulkiness (size), from smallest (top) to largest (bottom). tert- butyl group ethyl group fluorine atom hydrogen atom Rank the following substituents in increasing bulkiness (size), from smallest (top) to largest (bottom). Drag and drop options into correct order and submit. For keyboard navigation... SHOW MORE ✓ III = ||| ||| = = ||| tert-butyl group ethyl group fluorine atom hydrogen atomarrow_forward
- Please answer 4,8arrow_forwardPlease answer the following question.arrow_forwardWhat is the net ionic reaction for the titration of hydrobromic acid with sodium hydroxide? Group of answer choices OH−(aq) + HBr(aq) → Br−(aq) + H2O(l) OH−(aq) + H+(aq) → H2O(l) NaOH(aq) + HBr(aq) → NaBr(aq) + H2O(l) NaOH(aq) + H+(aq) → H2O(l) + Na+(aq)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY