
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
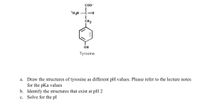
Transcribed Image Text:Co0-
*H3N -C-H
CH2
OH
Tyrosine
a. Draw the structures of tyrosine as different pH values. Please refer to the lecture notes
for the pKa values
b. Identify the structures that exist at pH 2
c. Solve for the pl
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give detailed Solution with explanation needed..don't give Handwritten answerarrow_forward3. Draw the form of the amino acid that predominates at pH 11. ( H₂N-CH- pKa = 9.60 CH₂ pKa = 8.95 c=0 OH pKa = 3.65 4. At what pH will the following amino acid no longer migrate in an electric field? H₂N-CH- (CH2)4 pKa = 8.95 NH₂ pKa = 10.53 H₂N-CH- C 5. Draw the form of the amino acid that predominates at pH 6. (CH2)4 O OH pKa = 1.88 C NH₂ pKa = 10.53 -OH pKa = 2. 18 -OH pKa = 2.18 6. All the following forces help to stabilize the tertiary and quaternary structures except: (a) Hydrophobic interactions of nonpolar side chains (b) Hydrogen bonding (c) Electrostatic interactions between ionic groups (d) Disulfide bonds (e) Metallic bondsarrow_forward1. Draw the structure of alanine in a solution at pH = 0 2. Draw the structure of alanine in a solution at pH = 12 3. Draw the structure of alanine in a solution at physiological pH (pH= 7.4)arrow_forward
- Draw the structure of the peptide WAKEP. Write the full name of each amino acid above the relevant R-group. Mark all of the peptide bonds on your drawing. Mark the charged groups on your drawing. Assuming that pH = 7, what is the Net Charge for the peptide? Type your numeric answer and submit -7 X You are incorrectarrow_forwardplease answer 1 and 2arrow_forwardball & stick This amino acid is Not started Submit Answer - + labels to access important values if needed for this question. Retry Entire Group 9 more group attempts remaining < ENG 6arrow_forward
- What would the dissociation equation for Histidine be if I were using these 3 different groups: carboxyl, amino, R group?arrow_forward9. For the DNA segment with nucleotide sequence 5' TATCGC 3’, how many hydrogen bonds in total hold the base pairs between this segment and its complementary strand? А. 5 В. 6 С. 15 D. 18 E. Cannot be determined 10. What is the relation between the Fischer projection of two molecules below? H H- -OH Но- но- H- Но H- H- -OH- Но- CH2OH CH2OH A. C2 epimers В. СЗ ерimers C. Enantiomers D. Anomers E. Superimposable molecules Section B: True / False (10 marks) A group is deprotonated when its pKa value is lower than the pH of the solution. 1. T or F 2. a and B configurations are used to describe enantiomers. T or F 3. Hydrogen bond can be formed between two peptide bonds. T or F 4. A single strand of RNA has 20% U so it also has 20% A. T or F 5. Arginine's pI lies between 2.34 and 9.6. T or F 6. O-6 fatty acids have fewer double bonds than @-9 fatty acids have. T or F 7. The net charge of glycine changes at different pH. T or F 8. The hydroxyl group in threonine is deprotonated at high pH. T…arrow_forwardSolve botharrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY