
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
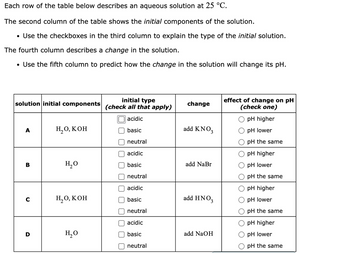
Transcribed Image Text:Each row of the table below describes an aqueous solution at 25 °C.
The second column of the table shows the initial components of the solution.
• Use the checkboxes in the third column to explain the type of the initial solution.
The fourth column describes a change in the solution.
• Use the fifth column to predict how the change in the solution will change its pH.
solution initial components
A
B
C
H₂O, KOH
H₂O
H₂O, KOH
H₂O
initial type
(check all that apply)
acidic
basic
neutral
acidic
basic
neutral
acidic
basic
neutral
acidic
basic
neutral
change
add KNO3
add NaBr
add HNO3
add NaOH
effect of change on pH
(check one)
pH higher
pH lower
pH the same
pH higher
pH lower
pH the same
pH higher
O pH lower
pH the same
pH higher
pH lower
pH the same
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- i.e. Determine the [H+] of a 500.0 mL solution that contains2.6 g of NaOHarrow_forwardI'm confused on how to do number 4 a and barrow_forwardThe anion of a strong acid a. is a neutral ion b. is an acidic ion C. is a basic ion d. will react with HT to establish equilibrium е. will produce H" ions in waterarrow_forward
- What 4 primary standards can be used to standardize acid solutions and their balanced reactions with HCLarrow_forwardEach row of the table below describes an aqueous solution at 25 °C. The second column of the table shows the initial components of the solution. • Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. • Use the fifth column to predict how the change in the solution will change its pH. initial type solution initial components (check all that apply) change effect of change on pH (check one) acidic pH higher H₂O A basic add KNO3 pH lower neutral pH the same acidic pH higher B H₂O, NaOH basic add HClO4 pH lower neutral pH the same acidic pH higher C H₂O basic add KOH pH lower neutral pH the same acidic pH higher H₂O, NaOH basic add NaC104 pH lower neutral pH the samearrow_forwardEach row of the table below describes an aqueous solution at 25 °C. The second column of the table shows the initial components of the solution. • Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. • Use the fifth column to predict how the change in the solution will change its pH. solution initial components initial type (check all that apply) change effect of change on pH (check one) acidic pH higher A H₂O basic add Nal pH lower neutral pH the same acidic pH higher B H₂O, KOH basic add H Br pH lower neutral pH the same acidic pH higher C H₂O basic add NaOH pH lower neutral pH the same acidic pH higher H2O, КОН basic add K Br pH lower neutral pH the samearrow_forward
- Select all chemical species present in the solution. i Multiple answers: Multiple answers are accepted for this question Select one or more answers and submit. For keyboard navigation... SHOW MORE ✓ a b C d NH4Cl(s) H₂O(l) 4+ (aq) NH4+ Cl(aq)arrow_forwardFor a certain acid pK = decimal places. pH = 0 4.49. Calculate the pH at which an aqueous solution of this acid would be 2.9% dissociated. Round your answer to 2 X 5arrow_forwardEach row of the table below describes an aqueous solution at 25 °C. The second column of the table shows the initial components of the solution. • Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. • Use the fifth column to predict how the change in the solution will change its pH. alo initial type (check all that apply) effect of change on pH (check one) solution initial components change O acidic pH higher н,о, Кон A basic add H Cl pH lower O neutral O pH the same O acidic O pH higher В Н,0, КОН O basic add K CI O pH lower neutral O pH the same O acidic O pH higher H,0 O basic add Na ClO, O pH lower O neutral O pH the same O acidic O pH higher H,0 O basic add Na OH O pH lower O neutral O pH the same Explanation Check © 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forward
- At 25oC, it took 25.8 mL of a 0.200 M NaOH solution to completely neutralize 32.00 mL of an unknown, diprotic acid solution. Determine the initial molarity of this unknown acid solution.arrow_forwardEach row of the table below describes an aqueous solution at 25 °C. The second column of the table shows the initial components of the solution. • Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. • Use the fifth column to predict how the change in the solution will change its pH. solution initial components initial type (check all that apply) effect of change on pH change (check one) A H₂O 0 000 0 acidic pH higher basic neutral acidic add K C104 pH lower B 80 H₂O, H Br basic neutral add Na Br O O acidic C H₂O, H Br basic neutral add NaOH pH lower acidic pH the same pH higher D H₂O basic add KOH pH lower neutral pH the same pH higher pH lower pH the same pH higher pH the samearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY