
Chemistry: Matter and Change
1st Edition
ISBN: 9780078746376
Author: Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher: Glencoe/McGraw-Hill School Pub Co
expand_more
expand_more
format_list_bulleted
Question
Please answer both of the fo
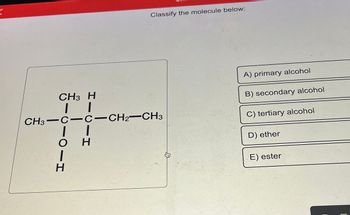
Transcribed Image Text:**Classify the Molecule**
**Molecule Structure:**
The image displays a structural formula of a molecule. Here is a breakdown of the structure:
- CH₃—C—CH₂—CH₃
- The central carbon (C) is bonded to a hydroxyl group (OH) and another hydrogen (H).
- The structure includes three methyl groups (CH₃), with one connected to the central carbon and two attached to adjacent carbons.
**Classification Options:**
A) Primary alcohol
B) Secondary alcohol
C) Tertiary alcohol
D) Ether
E) Ester
**Analysis:**
- The central carbon is connected to three other carbon atoms and has a hydroxyl group (OH) attached to it, indicating that this molecule is a tertiary alcohol.
**Correct Classification:**
C) Tertiary alcohol
![**Transcription for Educational Website:**
**Provide the abbreviation of the following nucleotide.**
[Image depicts a nucleotide structure, which includes a phosphate group linked to a ribose sugar, and an attached nitrogenous base.]
**Options to choose from:**
- d
- A
- G
- C
- T
- U
- MP
- DP
- TP
- deoxy
- ribose
- aden
- cyt
- guan
**Explanation of the Diagram:**
The diagram shows a chemical structure that includes three main components:
1. **Phosphate Group:** Represented by the chemical formula, connected to the sugar.
2. **Ribose Sugar:** A pentagon-shaped structure with hydroxyl (OH) groups.
3. **Nitrogenous Base:** This is a hexagonal ring structure, which specifies the type of nucleotide.](https://content.bartleby.com/qna-images/question/0e2724af-63d8-46e5-8bfa-efceefabbe5e/d4e423f3-e24a-49cc-84e8-745d9573b700/p85rsw_thumbnail.jpeg)
Transcribed Image Text:**Transcription for Educational Website:**
**Provide the abbreviation of the following nucleotide.**
[Image depicts a nucleotide structure, which includes a phosphate group linked to a ribose sugar, and an attached nitrogenous base.]
**Options to choose from:**
- d
- A
- G
- C
- T
- U
- MP
- DP
- TP
- deoxy
- ribose
- aden
- cyt
- guan
**Explanation of the Diagram:**
The diagram shows a chemical structure that includes three main components:
1. **Phosphate Group:** Represented by the chemical formula, connected to the sugar.
2. **Ribose Sugar:** A pentagon-shaped structure with hydroxyl (OH) groups.
3. **Nitrogenous Base:** This is a hexagonal ring structure, which specifies the type of nucleotide.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The physical and chemical properties of a molecule depend on its structure. Here are two ball-and-stick models for two compounds that have the same molecular formula but different structures and different chemical properties. Ethanol Dimethyl etherarrow_forwardExplain, in molecular terms, why oil and water do not mix.arrow_forward1. a) When calcium forms a compound with chlorine, electrons are transferred from the atom to the atom. b) What type of bonding exists between the calcium and chlorine atoms? c) Indicate the electrical charge on each of the ions and the total electrical charge on the compound. 2. What can be done to an ionic solid so that the ions are capable of motion? 3. How can we cause ions in a solution to move in a specific direction?arrow_forward
- Consider the following chart with ionic and covalent compounds shown. Ionic and Covalent Compounds Compound Melting Point ⁰C Boiling Point ⁰C W 801 1465 X 2852 3,600 Y 0 100 Z 113 184 Based on the data, which compounds would you classify as ionic or covalent and why? A Compound Y is ionic because its’ melting and boiling points are low. B Compound Y and Z are covalent because their melting and boiling points are low. C Compounds W, X, and Z are ionic because their melting and boiling points are high. D Compounds W, X, and Z are covalent because their melting and boiling points are high.arrow_forward1. Sodium, Neon, Oxygen and Argon are elements found on the periodic table Two of these elements that can react together to form a chemical compound. (a) What is the name and the formula of this compound? Name Formula (b) What type of bonding holds this compound together?arrow_forwardThe chemical formula of carbon tetrachloride is CCl4. The nature of this compound is?arrow_forward
- CCI, is a nonpolar molecule, while CHCI3 and CH2Cl2 are polar molecules. Draw the Lewis structures of these three molecules. Explain the observation in polarity of the molecules. 2.arrow_forwardWhat is the chemical formula for the compound formed between rubidium and fluorine? formula: What is the chemical formula for the compound formed between rubidium and selenium? formula:arrow_forwardDraw a skeletal ("line") structure of this molecule: OH O || CH -C-CH₂-CH3 CH3 || Show Transcribed Text G CI Ĉ Suppose all the chlorine atoms in this molecule are replaced by hydrogen atoms: Draw a skeletal ("line") structure of the new molecule.arrow_forward
- For the following ionic compounds, draw the Lewis symbols for the elements and, using arrows, show how electrons are transferred to make the ionic compounds. Also show the final ions formed and the formula of the ionic compound. a) aluminum chloride b) barium sulfidearrow_forwardPlease help me solve the following question and please please make sure everything is correct ! its important thanks !1arrow_forwardCite two examples of evidence that the name provides for EACH compound.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER  Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning