
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
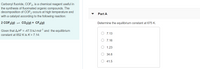
Transcribed Image Text:Carbonyl fluoride, COF2, is a chemical reagent useful in
the synthesis of fluorinated organic compounds. The
decomposition of COF2 occurs at high temperature and
with a catalyst according to the following reaction:
Part A
2 COF2(g) = CcO2(g) + CF4(g)
Determine the equilibrium constant at 675 K.
Given that A,HP = -47.5 kJ mol-1 and the equilibrium
constant at 852 K is K = 7.14:
7.13
7.16
1.23
O 34.6
41.5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Find the equilibrium constant K for this reaaction at 25C.arrow_forwardPlease solve ASAP Butene is obtained as a result of the dimerization reaction of ethylene: 2C2H4(g) → C4H8(g) Ethylene/Nitrogen mixture is fed to a reactor at 150 °C. Ethylene/Nitrogen mole ratio 0.5 is In the reaction with 75% conversion of ethylene, the products leave the reactor at 300 °C. If so, what is the heat of reaction?arrow_forwardodium nitrite is manufactured based on the reaction represented by the following chemical equation: Na2CO3(s) + 2NO(g) + O2(g) → 2 NaNO2(s) + CO2(g) The Na₂CO3 used is first dissolved in water to form a 40% solution. The NO and O2 used are 10% in excess of the theoretical requirement and the reaction is 90% complete. On a basis of 100 kg of Na₂CO3, calculate: a) the mass of NaNO2 obtained, b) the composition of the resulting solution (in mass percent), b) The composition of the issuing gas (in mole percentarrow_forward
- Please show the complete solution.arrow_forwardIf 50.0 g of H₂ and 125.5 g of O₂ react, how many moles of H₂O can be produced in the reaction below? 2 H₂(g) + O₂(g) → 2 H₂O(g)arrow_forwardWrite a balanced standard formation reaction and a balanced standard combustion reaction for each of the following compounds, and For each of the compounds in Q-9, calculate the standard enthalpy of combustion using enthalpies of formation from the table of thermodynamic properties a) CH4 (methane) b) C6H12O6 (s) [ glucose ]arrow_forward
- A chemical engineer is studying the following reaction: BF3(aq)+NH3(aq) → BF3NH3(aq) At the temperature the engineer picks, the equilibrium constant K, for this reaction is 1.1. The engineer charges ("fills") three reaction vessels with boron trifluoride and ammonia, and lets the reaction begin. She then measures the composition of the mixture inside each vessel from time to time. Her first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time she measures the compositions. reaction vessel compound concentration expected change in concentration BF 3 0.64 M O f increase OI decrease O (no change) A NH, 0.90 M O f increase OI decrease O (no change) BF;NH; 0.63 M O f increase O! decrease (no change) BF3 0.84 M O f increase O! decrease O (no change) В NH, 1.19 M O f increase OI decrease O (no change) BF;NH, 1.09 M O 1 increase OI decrease (no change) BF3 0.38 M O t increase OI decrease O (no change) NH, 0.64 M O t…arrow_forwardConsider the following dissolution of lead(II) fluoride (PbF>(s): O 5.9 kJ molr1 PbF>(s) - Pb2*(ag) + 2 F(ag) O 5.9 kJ mol1 Below is a table of thermodynamic quantities pertaining to this reaction: Submit Request Answer Substance AHP (kJ mol1) S° (J K-1 mol1) Part B PBF2(s) -664.0 110.5 Determine the standard entropy of reaction (A,S°) for the dissolution of lead(II) fluoride. Pb2*(aq) 0.9 18.5 O 119.6 J K1 mol1 F(ag) -335.4 -13.8 O -105.8 J K1 mor1 O 119.6 J K1 mor1 O 105.8 J K-1 mol1arrow_forwardA solid mixture containing MgSO4 is dissolved in water and treated with an excess of Ba(NO3)2, resulting in the precipitation of 0.6 g of BaSO4.The molecular weight of BaSO4 is 233 g/mol. Number of moles of MgSO4 in the mixture is? O 0.00257mol O 0.52700 mol O 0.05720 mol O 0.25700 mol O 0.02570 molarrow_forward
- For the following reaction at equilibrium in a reaction vessel, which one of the changes below would cause the Br2 concentration to decrease? 2NOBr(g) ↔ 2NO(g) + Br2(g) ΔH= 30 kJ/mol Increase the temperature Remove some NO Add more NOBr Compress the gas mixture into a smaller volumearrow_forwardBalance the following redox reaction in an acidic solution: UO22+(aq)+Zn(s)->U4+(aq)+Zn2+(aq)arrow_forward26.20 The data provided in Figure 26.7 are based on the diffusion of O₂ into SiO₂ formed from the oxidation of (100) crystalline silicon at 1000°C. Estimate the diffusion coefficient of O₂ in SiO₂ formed from the oxidation of (111) crystalline silicon at 1000 °C, using the data in the table below, provided by Hess (1990).* Time 1.0 2.0 4.0 7.0 16.0 Measured SiO₂ Film Thickness (um) 0.049 0.078 0.124 0.180 0.298 0.070 0.105 0.154 0.212 0.339 The maximum solubility of O₂ in the SiO₂ is 9.6-10-8 mole 0₂/cm³ solid at 1000°C and 1.0 atm O₂ gas partial pressure. *D.W. Hess, Chem. Eng. Education, 24, 34 (1990).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The