
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
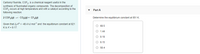
Transcribed Image Text:Carbonyl fluoride, COF,, is a chemical reagent useful in the
synthesis of fluorinated organic compounds. The decomposition of
COF, occurs at high temperature and with a catalyst according to the
• Part A
following reaction:
2 COF2(g) = CO2(g) + CFa(g)
Determine the equilibrium constant at 651 K.
Given that A,H° = -48.4 kJ mol-1 and the equilibrium constant at 821
Kis K= 9,17:
O 69.5
O 1.44
O 9.19
O 9.15
O 58.4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following equilibrium: 2NO, (g) = N,04 (g) AG° = - 5.4 kJ Now suppose a reaction vessel is filled with 8.21 atm of dinitrogen tetroxide (N,0,a) at 588. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N,0, tend to rise or fall? '2 fall Is it possible to reverse this tendency by adding NO,? In other words, if you said the pressure of N,O, will tend to rise, can that 4. yes be changed to a tendency to fall by adding N0,? Similarly, if you said the no pressure of N,04 will tend to fall, can that be changed to a tendency to rise by adding NO,? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO, needed to reverse it. atm Round your answer to 2 significant digits. O Oarrow_forwardA reaction has an equilibrium constant of 8000 at 298 K. At 785 K, the equilibrium constant is 0.54. Find ΔH∘rxnfor the reaction.arrow_forwardConsider the following equilibrium: N2 g) +3H, (g) =2NH3 (g) AG" = - 34. kJ Now suppose a reaction vessel is filled with 4.68 atm of nitrogen (N, and 2.51 atm of ammonia (NH,) at 260. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of NH, tend to rise or fall? fall Is it possible to reverse this tendency by adding H,? In other words, if you said the pressure of NH, will tend to rise, can that yes be changed to a tendency to fall by adding H,? Similarly, if you said the no pressure of NH, will tend to fall, can that be changed to a tendency to rise by adding H2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H, needed to reverse it. || atm Round your answer to 2 significant digits. O Oarrow_forward
- FaceboOK [Review Topics] [References) Use the References to access important values if needed for this question. Consider the following system at equilibrium where AH° 268 kJ, and K. 5.10x106, at 548 K. %3D NH,CI(s) NH3(g) + HCI(g) When some moles of NH,CI(s) are removed from the equilibrium system at constant temperature: The value of K The value of Q. The reaction must O run in the forward direction to restablish equilibrium. O run in the reverse direction to restablish equilibrium. O remain the same. It is already at cquilibrium. The concentration of NH, will Retry Entire Group 1 more group attempt remaining Submit Answerarrow_forward3. Consider the following reaction: Co(g) + 2H2(g) S CH;OH(g); Kp = 2.26x10ª at 25 °C Calculate AG for the reaction at 25 °C for each of the following conditions: (a) standard conditions (b) at equilibrium (c) P (CH3OH) = 1.0 atm, P (CO) = P (H2) = 0.010 atmarrow_forwardConsider the following system at equilibrium where K, = 154 and AH° = -16.1 kJ/mol at 298 K. 2 NO (g) + Brz (g) =2 NOB (g) The production of NOB (g) is favored by: Indicate True (T) or False (F) for each of the following; | 1. increasing the temperature. 2. increasing the pressure (by changing the volume). | 3. increasing the volume. V 4. removing NOB . 5. adding Br2.arrow_forward
- A2(g) + 3B2(g) -> 2AB3(g) where the ΔGorxn is -140 kJ and equilibrium constant at standard conditions is 3.5x1024. a) If we place 0.100 atm of AB3, 0.050 atm of B2, and 0.500 atm of A2 in a container at 25oC, which direction will the reaction go (if any) to reach equilibrium? b) What is the value of ΔGrxn assuming the temperature is maintained at 25oC?arrow_forwardUse thermochemical data given in the table to decide whether the equilibrium constant for each of the following reactions will increase or decrease with temperature. Substance AH; (kJ/mol) CO(g) CO₂ (g) CS₂(g) CH₁ (9) H₂(g) H₂S(g) H₂O(g) N₂ (9) NH3 (9) O₂(g) -111 -394 117 -74.9 0 -20.5 -242 0 -45.9 0 a. CO₂(g) + H₂(g) → CO(g) + H₂O(g) O The equilibrium constant will increase with temperature increase. O The equilibrium constant will decrease with temperature increase. b. 2CO2 (g) 2CO(g) + O2(g) O The equilibrium constant will increase with temperature increase. O The equilibrium constant will decrease with temperature increase.arrow_forwardA] HgS occurs in two crystalline forms called "red" and "black". For the conversion of 8. L- the red form to the black form at 525 °C, 4Gº = -0.157 kJ mol-¹ and AH = 4.184 kJ mol-¹. Assuming the AH is temperature independent, find the temperature at which the two forms can coexist at equilibrium at 1.000 bar. Which is more stable above this temperature?arrow_forward
- Consider the following system at equilibrium where Ho = 18.8 kJ, and Kc = 9.52E-2, at 350 K: CH4(g) + CCl4(g) 2 CH2Cl2(g) If the VOLUME of the equilibrium system is suddenly decreased at constant temperature: whats the value of Kc The value of Q The reaction mustarrow_forward[Review Topics] [References] Use the References to access important values if needed for this question. Consider the following system at equilibrium where AH=-108 kJ, and K. 77.5, at 600 K. CO(g) + C,(g) COCI,(g) When 0.22 moles of Cl,(g) are added to the equilibrium system at constant temperature: The value of K The value of Qe K. The reaction must O run in the forward direction to restablish cquilibrium. O run in the reverse direction to restablish cquilibrium. O remain the same. It is already at cquilibrium. The concentration of CO will Submit Answer Retry Entire Group 1 more group attempt remainingarrow_forwardSome chemical compounds are listed in the first column of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. The important chemical species that would be present in this solution are written in the second column of the table. Use the checkboxes to classify each compound. compound HCI W Ba (OH), HC,H,O, KCI Explanation patric(most common) 1 Other place . с important species present when dissolved in water E н,о, ст. н.о Ba, OH, H₂O но, с.н,о.. нс.н, о,, H,о K, CI, H₂O Check $ 4 R type of compound (check all that apply) ionic molecular strong weak strong weak acid acid base base Speciation is happening because the two groups split and live in other places 0 % 5 0 0 0 0 0 T 0 0 0 G Search or type URL 0 0 0 0 X Y 0 0 0 0 S & 0 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Access 0.5 U * 00 8 9arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY