
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
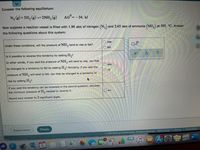
Transcribed Image Text:Consider the following equilibrium:
N, (g) + 3H, (g) = 2NH, (g)
AG=-34. kJ
Now suppose a reaction vessel is filled with 1.96 atm of nitrogen (N,) and 2.65 atm of ammonia (NH,) at 505. °C. Answer
the following questions about this system:
do
rise
Under these conditions, will the pressure of NH, tend to rise or falI?
fall
Is it possible to reverse this tendency by adding H,?
In other words, if you said the pressure of NH, will tend to rise, can that
yes
be changed to a tendency to fall by adding H,? Similarly, if you said the
no
pressure of NH, will tend to fall, can that be changed to a tendency to
rise by adding H,?
If you said the tendency can be reversed in the second question, calculate
the minimum pressure of H, needed to reverse it.
atm
Round your answer to 2 significant digits.
Explanation
Check
Terms of Use
Privacy Accessibility
©2021 McGraw-Hill Education. All Rights Reserved.
o aftmuPAnp an eaPuAL CamtPIL adninu safl cauOud.va nLIOMIPaM aidune anans.amnain.
W
O O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the reaction: CO(g) + Cl₂(g) COC12 (g) € Kp At equilibrium, this reaction is At equilibrium, the rates of→ and reaction are equal. At equilibrium, reactant and product concentrations are constant. Reactions continue after equilibrium has been reached. Decreasing the temperature changes the K, value for this reaction. Removing COC1₂(g) changes the value of K, for this reaction. For the reaction: COCI, (g) = CO(g) + Cl₂(g), the value of K, is equal to 6.7 x 10¹1 at 25°C V Varrow_forwardConsider the following equilibrium: 2NH₂ (g)-N₂ (g) + 3H₂(g) AG-34. KJ Now suppose a reaction vessel is filled with 4.85 atm of ammonia (NH3) a system: Under these conditions, will the pressure of H, tend to rise or fall? Is it possible to reverse this tendency by adding N₂? In other words, if you said the pressure of H, will tend to rise, can that be changed to a tendency to fall by adding N₂? Similarly, if you said the pressure of H₂ will tend to fall, can that be changed to a tendency to rise by adding N₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of N₂ needed to reverse it. Round your answer to 2 significant digits. and 2.40 atm of hydrogen (H₂) at 658. °C. Answer the following questions about this Ⓒrise O fall yes no atm 10 10 X Sarrow_forwardConsider the following system at equilibrium where H° = -111 kJ/mol, and Kc = 0.159, at 723 K. N2(g) + 3H2(g) 2NH3(g) If the TEMPERATURE on the equilibrium system is suddenly decreased: The value of Kc fill in the blank 1 A. increases. B. decreases. C. remains the same. The value of Q fill in the blank 2 A. is greater than Kc. B. is equal to Kc. C. is less than Kc. The reaction must: fill in the blank 3 A. run in the forward direction to reestablish equilibrium. B. run in the reverse direction to reestablish equilibrium. C. remain the same. It is already at equilibrium. The concentration of H2 will: fill in the blank 4 A. increase. B. decrease. C. remain the same.arrow_forward
- If Q is greater than K, the system will shift to the right to attain equilibrium. true or false?arrow_forwardConsider the following equilibrium: N₂(g) + 3H₂(g)2NH3(g) AG = -34. KJ Now suppose a reaction vessel is filled with 3.40 atm of hydrogen (H₂) and 9.24 atm of ammonia system: Under these conditions, will the pressure of NH3 tend to rise or fall? Is it possible to reverse this tendency by adding N₂? In other words, if you said the pressure of NH3 will tend to rise, can that be changed to a tendency to fall by adding N₂? Similarly, if you said the pressure of NH3 will tend to fall, can that be changed to a tendency to rise by adding N₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of N₂ needed to reverse it. Round your answer to 2 significant digits. rise O fall yes Ono X (NH3) at 736. °C. Answer the following questions about this Sarrow_forwardHeating HI(g) at 425 °C causes some of this compound to decompose, forming H2 (g) and I2 (g). Eventually, the amounts of the three species do not change further, the system has reached equilibrium. (At this point, approximately 22% of the HI has decomposed.) What is happening in this system at the molecular level? O H2(g) reacts with I2 (g) to produce HI(g) at the same rate as it decomposes. H2 (g) and I2 (g) molecules collide with HI(g) making the reaction stop. Only part of the HI(g) molecules has enough energy to decompose at this temperature. 5:55 PM FLV 2/22/2020 Type here to search hp ins prt sc delete home f12 f8 f9 f10 f5 f6 f7 14 10 num & backspace lock 23 8. 3 %24arrow_forward
- Consider the following reaction:COCl2(g) CO(g) + Cl2(g)If 2.98×10-2 moles of COCl2, 0.206 moles of CO, and 0.304 moles of Cl2 are at equilibrium in a 17.8 L container at 672 K, the value of the equilibrium constant, Kp, is .arrow_forwardConsider the equilibrium, 2 NO (g) N2₂ (g) + O₂ (g), 2 for which AH = -180 kJ mol-1. How will adding more N₂ (g) affect an equilibrium mixture of the three gases? Select one: a. Reaction will proceed to the right (in the forward direction) to generate more product. b. Reaction will proceed to the left (in the reverse direction) to generate more product. C. Reaction will proceed to the right (in the forward direction) to generate more reactant. d. Reaction will proceed to the left (in the reverse direction) to generate more reactant. e. There will be no change to equilibrium mixture.arrow_forwardConsider the following equilibrium: 2NO, (g)–N,04 (8) AGº= – = - 5.4 kJ Now suppose a reaction vessel is filled with 5.06 atm of dinitrogen tetroxide (N,0) at 400. °C. Answer the following questions about this system: 2 rise Under these conditions, will the pressure of N,0, tend to rise or fall? 2 4 fall Is it possible to reverse this tendency by adding NO,? In other words, if you said the pressure of N,0, will tend to rise, can yes that be changed to a tendency to fall by adding NO,? Similarly, if you no said the pres of N,0, will tend to fall, can that be changed to a 2 4 tendency to rise by adding NO,? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO, needed to reverse it. atm Round your answer to 2 significant digits.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY