
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
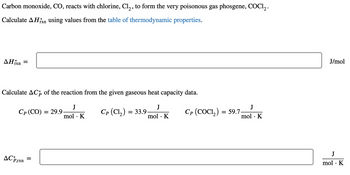
Transcribed Image Text:Carbon monoxide, CO, reacts with chlorine, Cl₂, to form the very poisonous gas phosgene, COC1₂.
Calculate AHin using values from the table of thermodynamic properties.
rxn
AHixn
=
Calculate ACp of the reaction from the given gaseous heat capacity data.
J
mol. K
J
mol. K
Cp (CO) = 29.9.
ACP,rxn
=
Cp (Cl₂) = 33.9.
Cp (COCI,) = 59.7
J
mol. K
J/mol
J
mol. K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In a reaction, 1.00 mole of H2 is produced at a constant pressure of 1.00 atm and at 298 K (1 L atm = 101.3 J): 2 H2O(g) → 2 H2(g) + O2(g) ΔH° = +483.6 kJ mol–1 How much heat is exchanged in this reaction? +483.6 kJ +967.2 kJ +241.8 kJarrow_forward33.6 g of a salt solution was mixed with 27.5 g of water in a calorimeter with a calorimeter constant of 66.5 J K-1 with all substances initially at 30.9 °C. The resulting solution was observed to be at a temperature of 12.2 °C and have a heat capacity of 3.97 J g K-1. Determine q for the mixing process. Oa. -5780 J Ob. 4540 J Oc. 8.05 x 104 J Od. 5780 J Oe. -7.86 x 104 Jarrow_forwardThe thermite reaction, used for welding iron, is the reaction of FegO4 with Al. 8 Al (s) + 3 Fe3O4 (s) > 4 Al203 (s) + 9 Fe (s) AH° = -3350 kJ Because this large amount of heat cannot be rapidly dissipated to the surroundings, the reacting mass may reach temperatures near 3000°C. How much heat (in kJ) is released by the reaction of 16.5 g of Al with 76.7 g of Fe304? Enter your numerical answer in units of kJ.arrow_forward
- Under what conditions does q, the heat evolved or absorbed by the system in a physical or chemical process, equal the change in enthalpy of the system? 1. When q = 0. 2. When w = 0. 3. When w = -PAV. O 1, 2, and 3 O 1 only 3 only O 2 only O 2 and 3arrow_forwardular receptors pid-eoluble structure amembrane to an ptor to the receptor n from the ression Name Problems: Experiment 2 You must show calculations in the space provided and place the answer on the line to receive credit. 1. A 64.525 g sample of metal at 100.0 °C was placed in 43.635 g of water at 24.6°C. At equilibrium the temperature of the water and metal was 36.8 °C. a) Calculate the At of the waterarrow_forward1. A 50g sample of metal iron that was heated to 90 °C was dropped into a coffee cup calorimeter containing 200mL of water at room temperature of 25 °C. Applying the density of water (1g/mL) and specific heat capacity capacities of water and iron, determine the final temperature when the system reaches equilibrium.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY