
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
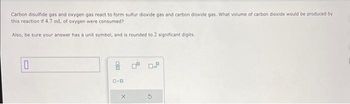
Transcribed Image Text:Carbon disulfide gas and oxygen gas react to form sulfur dioxide gas and carbon dioxide gas. What volume of carbon dioxide would be produced by
this reaction if 4.7 mL of oxygen were consumed?
Also, be sure your answer has a unit symbol, and is rounded to 2 significant digits.
10
pla
8
ロ・ロ
0.9
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A major component of gasoline is octane (C,H). When octane is burned in air, it chemically reacts with oxygen gas (O,) to produce carbon dioxide (CO,) and water (H,O). What mass of oxygen gas is consumed by the reaction of 7.8 g of octane? Round your answer to 2 significant digits.arrow_forwardSodium Chloride was placed in a beaker and the beaker and the sodium chloride together had a mass shown below. If the mass of the empty beaker is 47.724g. What is the mass of the NaCl in grams? Your answer must be exactly correct and expressed to the correct number of significant digits. -80 100F ml -40 54.424 garrow_forwardGaseous ethane CH3CH3 will react with gaseous oxygen O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O. Suppose 19. g of ethane is mixed with 52.1 g of oxygen. Calculate the minimum mass of ethane that could be left over by the chemical reaction. Be sure your answer has the correct number of significant digits.arrow_forward
- A chemist determines by measurements that 0.0500 moles of oxygen gas participate in a chemical reaction. Calculate the mass of oxygen ges that participates Be sure your answer has the correct number of significant digits. D.P X dlaarrow_forwardMethane gas and chlorine gas react to form hydrogen chloride gas and carbon tetrachloride gas. What volume of hydrogen chloride would be produced by this 3 reaction if 6.0 cm° of methane were consumed? Also, be sure your answer has a unit symbol, and is rounded to 2 significant digits. x10 미□arrow_forwardMethane gas and oxygen gas react to form water vapor and carbon dioxide gas. What volume of water would be produced by this reaction if 2.6 mL of methane were consumed? Also, be sure your answer has a unit symbol, and is rounded to the correct number of significant digits. 0|0 X Ś x10arrow_forward
- Carbon disulfide gas and oxygen gas react to form sulfur dioxide gas and carbon dioxide gas. What volume of carbon dioxide would be produced by this reaction if 1.22cm^3 of oxygen were consumed? Also, be sure your answer has a unit symbol, and is rounded to the correct number of significant digits.arrow_forwardHydrogen gas and nitrogen gas react to form ammonia gas. What volume of ammonia would be produced by this reaction if 5.99 cm of hydrogen were consumed? Also, be sure your answer has a unit symbol, and is rounded to 3 significant digits.arrow_forwardMethane gas and chlorine gas react to form hydrogen chloride gas and carbon tetrachloride gas. What volume of hydrogen chloride would be produced by this reaction if 9.94 m² of methane were consumed? Also, be sure your answer has a unit symbol, and is rounded to 3 significant digits. 0 8 0.0 X 0x10 5 ? O 000 Ararrow_forward
- 13 A major component of gasoline is octane (C₂H₁). When liquid octane is burned in air it reacts with oxygen (O₂) gas to produce carbon dioxide gas and water vapor. Calculate the moles of oxygen needed to produce 0.70 mol of water. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits. 0 80.² 0.0arrow_forwardAmmonia gas and oxygen gas react to form water vapor and nitrogen monoxide gas. What volume of water would be produced by this reaction if 5.33 mL of ammonia were consumed?Also, be sure your answer has a unit symbol, and is rounded to 3 significant digits.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY