
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
can you type this out in word using the equation function i cant understand this at all
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 4 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
great can u help me w/ part C now?
Calc the V of reactor for 90% conversion
Solution
by Bartleby Expert
Follow-up Question
awesome not that you helped me solve part A can you help me solve part B
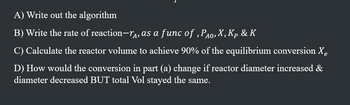
Transcribed Image Text:A) Write out the algorithm
B) Write the rate of reaction-, as a func of, PÃO, X, Kp & K
C) Calculate the reactor volume to achieve 90% of the equilibrium conversion Xe
D) How would the conversion in part (a) change if reactor diameter increased &
diameter decreased BUT total Vol stayed the same.
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
great can u help me w/ part C now?
Calc the V of reactor for 90% conversion
Solution
by Bartleby Expert
Follow-up Question
awesome not that you helped me solve part A can you help me solve part B
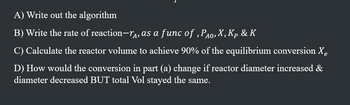
Transcribed Image Text:A) Write out the algorithm
B) Write the rate of reaction-, as a func of, PÃO, X, Kp & K
C) Calculate the reactor volume to achieve 90% of the equilibrium conversion Xe
D) How would the conversion in part (a) change if reactor diameter increased &
diameter decreased BUT total Vol stayed the same.
Solution
by Bartleby Expert
Knowledge Booster
Similar questions
- Discuss the difference of undo and redo in AutoCAD. Minimum of 5 Sentences.arrow_forwardUsing the Kroghtissue cylinder model learned in class, we intend to calculate the glucose concentration around the blood vessels in the muscle tissue. (For calculation, it is assumed that the vessel has the following conditions in images.) Calculate C(rT, z) when r=rT and draw a graph with the value of z as the x-axis.arrow_forwardHelp!!!arrow_forward
- Q1/If the mercury thermometer is used as a measuring device in a control system. What is the degree of it? And show how to reduce it's time constant? Then, if this thermometer having a time constant of (30 x 106nh) is initially at (560 °C), it is immersed in a bath at (150 °C) at (t=0), determine the indicated temperature at (t=3.5 min). Also, if the thermometer is read a temperature at (t =4.5 min), and then is return to the initial bath, find what will be the temperature reading at (t =6.5 min)?arrow_forward(a) Identify the temperature, composition, and phase transformation equation for each eutectic/eutectoid/peritectic/peritectoid point shown in plot b) In real world applications, there is often a need for the production of steel/iron systems that have diverse properties because Fe-based alloy systems tend to be very cheap and have high strength. Identify a composition and temperature that you would use to obtain the following materials, then calculate the percentage of each phase present at the point in the diagram and the percentage of Fe and C in each phase. You may need to use the internet to obtain some generic (but readily available) information about the properties of each phase i. A steel with no ductile-to-brittle transition temperature (DBTT) and is non-magneticii. A steel containing 2 metallic phases but no ceramic phases iii. A cast iron containing at least 40% cementite iv. A magnetic steel with an 100% eutectic or eutectoid structure (c) Many stainless steels (containing…arrow_forwardPlease solve clearly, Im needed in 30-60 minutes thank u Find Linear Algebra applications in the field of Chemical Engineering studiarrow_forward
- I apologize upfront, but I do not understand the solutions here. I cannot follow along due to the way it is typed. I've tried writing it out many times and never come to the same answers as you. Is it possible to get these solutions hand written?arrow_forward22 prove that the following flow chart depicts a process by writing thre balanced balances. 0.2 -8 Ibm Oz/ik To lbm 741bm Hs I I bm Ozarrow_forwardthis is great, can you please type out solution using word (equation) its hard for me to readarrow_forward
- PLEASE ANSWER QUESTION WITH RIGHT ANSWERS, ANSWERS ATTACHED FEEDBACK ATTACHED ALSO DONT COPY AND PASTE FROM OTHER ANSWERED QUESTIONS AS THESE ARE WRONGarrow_forwardThe following data represent the color of men's dress shirts purchased in the men's department of a large department store. Construct a category frequency distribution for the data (W = white, BL = blue, BR = brown, Y = yellow, G = green). W W BR Y BL BL W W Y G W W BL BR BL BR BL BL BR Y BL W BL W W BL W BL BR Y BL BR G BR W W BR Y W BL Y W W BL W BR G Garrow_forwardThe problem that is listed below need to be solved and you may access that problem via viewing them through the attached images in this request. **Question Number #2.15**arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The