
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
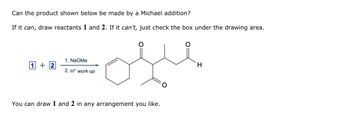
Transcribed Image Text:Can the product shown below be made by a Michael addition?
If it can, draw reactants 1 and 2. If it can't, just check the box under the drawing area.
1. NaOMe
1+2
2. H+ work up
ofle
You can draw 1 and 2 in any arrangement you like.
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Be sure to answer all parts. Draw a stepwise mechanism for the following reaction. Part 1 out of 4 OH 2 attempts left HBr Draw the product of the first step, be sure to include lone pairs and charges. OH Check my work + Br HBr l.no + H₂O draw structure ... Next part i+ Brarrow_forwardOResources x Give Up? Predict the two major organic products of the reaction. (HI behaves as an HX reagent.) Draw the two major organic products. Draw Rings More Erase Select H .CH3 CH3 CH, H + H H. CH, H-Iarrow_forward2 please help with homeworkarrow_forward
- Sent 3. Use curved arrows to show the electron pushing mechanism for the following reaction: OH H30* HOarrow_forwardreagent. alio 1.) Circle the following molecules that can be made into a Grignard herd HO Br Br CI o O CI Br = Brarrow_forward4. The following two compounds are protonated when treated with one mole equivalent of a strong acid (e.g. HCI). For each compound, draw the equilibrium to show the preferred site of protonation and give an estimate of the base strength (pKaH). -OHarrow_forward
- Can the product shown below be made by a Michael addition? If it can, draw reactants 1 and 2. If it can't, just check the box under the drawing area. 1. NaOMe +2 2. H+ work up CN You can draw 1 and 2 in any arrangement you like.arrow_forwardQuestion #9arrow_forwardDraw the major product for this reaction. Ignore inorganic byproducts. 1. Hg(OAc)2, H2O 2. H2SO4 %3D Drawing Atoms, Bonds and Rings Charges Draw or tap a new bond to see smart suggestions. Undo Reset Remove Donearrow_forward
- Draw the major organic product or products for the reaction. Multiple products may be drawn in one box, in any order. Include charges as needed. F. F F HNO3, H₂SO4 The \\(\ce {NO2} \\) can be added from the Groups menu. Draw Templates Groups More Select C H F Erasearrow_forwardConsider the addition of hydrogen chloride to (E,E)-2,4-hexadiene. a. Draw the bond-line formula (or skeletal structure) of the 1,2-addition product. b. Draw the bond-line formula (or skeletal structure) of the 1,4-addition product.arrow_forwardDraw the product(s) of the reaction below. O: H + Add/Remove step Х Click and drag to start drawing a structure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY