
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Can someone draw the entire mechanism with arrows for this reaction? I am mainly confused regarding two things:
1) The free radical (Br2/hv) addition of the bromine to cyclohex-1,4-diene
2) The addition of the
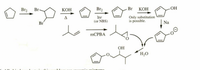
Transcribed Image Text:Br2
Br-
КОН
Br2
Br
КОН
ОН
hv
(or NBS)
Only substitution
is possible.
Br
Na
do
mCPBA
ОН
H2O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following reaction. CH3CH₂CH=CHCOH HCI CH3CH₂CH CHCOH c₁ CH3CH₂CH- H O CHCOH H CI this product is not observed Account for the fact that this reaction yields only a single product by drawing the carbocation intermediate leading to the observed product.arrow_forwardProvide mechanistic pathway for each step and the products based on the reagents selected. Diels Alders reactions to consider the formation of the products stated below. Product in step 1 is used for step 2. Product instep 2 is used for step 3. Indicate any required stereochemistry (E, Z, R, S).Step 1: 3-bromo-3-methylbut-1-ene with sodium ethoxideStep 2: addition of maleic anhydride and heatStep 3: hydrogen gas bubbling with palladium metalarrow_forwardWhat is the major product for each of the following reactions? (If an enol is created, write both the enol and keto products. If both ortho and para products are made, write both.)arrow_forward
- When an unsymmetrical Alkenes such as propane is treated with N-bromosuccinimide in aqueous dimethyl sulfoxide, the major product has the bromine atom bonded to the less highly substituted carbon atom. Is this Markovnikov or non-Markovnikov orientation? Show its complete reaction mechanism.arrow_forwardConsider the attached SN2 reaction. Question: What happens to the reaction rate in each of the following instances? [1] The leaving group is changed from Br− to I−; [2] The solvent is changed from acetone to CH3CH2OH; [3] The alkyl halide is changed from CH3(CH2)4Br to CH3CH2CH2CH(Br)CH3; [4] The concentration of −CN is increased by a factor of five; and [5] The concentrations of both the alkyl halide and −CN are increased by a factor of fivearrow_forwardSelect the correct arrow-pushing mechanism for the formation of the more stable carbocation intermediate in he addition of a hydrogen halide to the following alkene. H-Br: O H-Br: + Br H-Br: H + :Br H-Br: Brarrow_forward
- Does a rotation about the carbon-carbon sigma bond always happen in a E1 reaction? If not, why is it happening in this reaction?arrow_forwardThe Wolff–Kishner reaction uses hydrazine (H2NNH2) and hydroxide (–OH) to reduce a carbonyl to the alkane. The first steps of the mechanism convert a carbonyl to a hydrazone in a manner similar to imine formation. Draw the mechanism arrows for the reaction from the hydrazone to the alkane. Be sure to add lone pairs of electrons and nonzero formal charges to all species.arrow_forwardol The conjugate addition of an alkyl group to an a,ß-unsaturated ketone is one of the more useful 1,4-addition reactions, just as direct addition of a Grignard reagent is one of the more useful 1,2-addition reactions. Conjugate addition occurs via the use of a lithium dialkylcopper reagent, also called a Gilman reagent. The mechanism is thought to involve the addition of the dialkylcopper anion to the B- carbon to give a copper-containing intermediate. Transfer of an alkyl group to carbon and release of a neutral alkylcopper species completes the reaction. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions سلم NOC XT Ph :0: CH₂ 1. (CH3CH₂)2CuLi 2. H₂O* CH₂CH3 Cu CH₂CH3 Ph Cu CH3 CH₂CH3 CH₂CH3 8aarrow_forward
- Draw the ALL of the E2 organic product formed when the structure shown below undergoes dehydrohalogenation in alcoholic KOH with heat. Which of the products is formed the most?arrow_forwardTreatment of 1,2-dibromoethane with the dithiolate dianion shown in the reaction below leads to two products as shown. Draw the structure for each molecular formula and provide a detailed mechanism for the formation of both products.arrow_forwardThe following two reactions are performed on the same starting material; one producing “product 1" and one producing "product 2" as the major products. Which of the following statements is false regarding these two reactions? Both of these reactions proceed A via a carbocation intermediate. Product 1 is a substitution В product. The leaving group in the reaction producing product 2 is H20. C Product 2 is a disubstituted alkene. Both of these reactions proceed E via a unimolecular reaction mechanism. он HCI (conc.) Product 1 (Major) он H2SO4 (conc.) Product 2 (Major)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY