
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
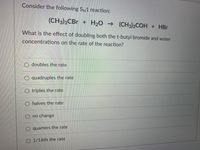
Transcribed Image Text:Consider the following SN1 reaction:
(CH3)3CBR + H2O → (CH3)3COH + HBr
What is the effect of doubling both the t-butyl bromide and water
concentrations on the rate of the reaction?
O doubles the rate
O quadruples the rate
O triples the rate
O halves the rate
O no change
O quarters the rate
O 1/16th the rate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the mechanism for the reaction shown. H2 N. N. CI CI 2.arrow_forwardYou are working in a chemistry laboratory and want to determine the mechanism for the following reaction: > CH3CH₂CH₂OH + Br CH3CH₂CH₂Br + ¯OH --arrow_forwardHow do you draw the mechanism for the last reaction (with phosphorus and I2)?arrow_forward
- The rate of the following reaction is dependent on the concentration of: Br + NaN3 DMF N3 + NaBrarrow_forwardWhat amount of catalyst is necessary to significantly change the reaction rate of peroxide hydrogen decomposition? O A catalyst does not influence the rate of reaction O Equal to hydrogen peroxide O Half of hydroxide peroxide O Small DD 000 החמ F9 F10arrow_forward03 AllC :Draw the Mechanism (stepwise) of any ONE of the following reactions Ag(I) 1. OH H,SO4 NH 2. I B ENG 4) G T-T/-E/-arrow_forward
- species A converts into a product species E. Energy A B Exothermic and A Exothermic and B Exothermic and C A E C 2 D Reaction Progress B is rate determining. C is rate determining. D is rate determining. 3h in which a reactant Is the overall reaction exothermic or endothermic? And which step is the rate determining step? Earrow_forwardMechanism for this reaction?arrow_forwardWhich arrow best describes the mechanism for the reaction? B C D O A 台灣券 O B OD 2. SI O-0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY