
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
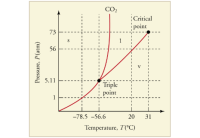
Can carbon dioxide be liquefied at room temperature ( 20ºC )? If so, how? If not, why not? (See Figure.)
Figure: The phase diagram for carbon dioxide. The axes are nonlinear, and the graph is not to scale. Dry ice is solid carbon dioxide and has a sublimation temperature of – 78.5ºC .
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 3 of 4 A stainless-steel-bottomed kettle, its bottom 25 cm in diameter and 1.6 mm thick, sits on a burner. The kettle holds boiling water, and energy flows into the water from the kettle bottom at 800 W. Review I Constants I Periodic Tabl Part A What is the temperature of the bottom surface of the kettle? Thermal conductivity of stainless steel is 14 W/(m K). Express your answer using four significant figures. VO AEO ? T = 102.65 °C Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Review your calculations and make sure you round to 4 significant figures in the last step. Ne Provide Feedbackarrow_forwardShow ALL pertinent solutions. Tabulate data of iteration after the solution.arrow_forwardA two phase liquid - vapor mixture of refridgerant a2 is Contained in a ciosed tank at 10 bar. The quality is l60% and the mass of saturated liquid is 25 kg. Calculate the volume of saturated vapor in m*. Use 5 signiigant figures.arrow_forward
- The image shows the example of finding the number of vacancies in 1 cubic meter of copper (Cu) at 1000 degrees celcius (1273 k) considering the image data. Replicating the problem in the image, calculate the number of vacancies but at room temperature.Explain why there is such a difference in the number of vacancies at both temperatures.arrow_forwardConstants Periodic Table Assume all temperatures to be exact, and neglect significant figures for small changes in dimension. On a warm day (92 °F), an air-filled balloon occupies a volume of 0.300 m³ and has a pressure of 24.0 lb/in?. Part A If the balloon is cooled to 32 °F in a refrigerator while its pressure is reduced to 14.7 lb/in?, what is the volume of the air in the container? (Assume that the air behaves as an ideal gas.) nνα ΑΣφ V = 0.170 m3 Submit Previous Answers Request Answer X Incorrect; Try Again; 6 attempts remainingarrow_forwardLearning Goal: To understand the ideal gas law and be able to apply it to a wide variety of situations. The absolute temperature T, volume V, and pressure p of a gas sample are related by the ideal gas law, which states that PV = nRT Here n is the number of moles in the gas sample and R is a gas constant that applies to all gases. This empirical law describes gases well only if they are sufficiently dilute and at a sufficiently high temperature that they are not on the verge of condensing. In applying the ideal gas law, p must be the absolute pressure, measured with respect to vacuum and not with respect to atmospheric pressure, and I must be the absolute temperature, measured in kelvins (that is, with respect to absolute zero, defined throughout this tutorial as -273°C). If p is in pascals and V is in cubic meters, use R = 8.3145 J/(mol · K). If p is in atmospheres and V is in liters, use R = 0.08206 L atm/(mol-K) instead. Part A A gas sample enclosed in a rigid metal container at…arrow_forward
- An ideal gas is confined to a constant volume container and heated from a pressure of 1 atm at room temperature to a pressure of 5 atm. What is the final temperature. Thank you for the help. I got 297K but I do not think that is it.arrow_forwardCan you please solve the below problem, showing step by step: A balloon contains mostly helium and a little nitrogen. The pressure of the helium is 425 mm Hg. What is the partial pressure of the nitrogen if the total pressure of the balloon is 67.98 kPa?arrow_forwardThis is a calculation only. Energy2d isn’t able to do phase changes as far as I’ve learned. There was a different simulation for this but it is not currently free. You combine 50g of ice at -10oC with 150g of liquid water at 75oC. After a few minutes, you measure the final equilibrium temperature of all of the H2O to be 35.1 oC. Use this information to “experimentally” determine the heat of transformation of water, Lfusion. cwater=4190 J/kgK, cice=2090 J/kgK Show all of your work and compare to the accepted value Lfusion=3.33x105 J/kg.arrow_forward
- A tank of N2 gas has temperature 500 K. Calculate the percentation of N2 molecules that has a speed between 1000 m/s and 2000 m/s. ____% 2 sig. fig. Please solve in Excel numerically the Maxwell-Boltzmann distributionarrow_forwardYou are studying a liquid that you have just synthesized in the laboratory, and you would like to get a rough idea of its enthalpy of J -), you measure its normal boiling point and find that it is mol. K vaporization. Assuming that it reflects Trouton's rule (namely that A vap 368 K. Use this result to estimate AvapH in kJ/mol. S≈ 88-arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON