
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
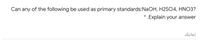
Transcribed Image Text:Can any of the following be used as primary standards:NaOH, H2SO4, HNO3?
.Explain your answer
إجابتك
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can I please have the answers as drawings for thesearrow_forwardSolid calcium carbonate, CaCO3, was added to a solution of pure water. Analysis found 1.20*10-3 M Ca2+ ions in solution. Calculate the Ksp of CaCO3. Be sure to use 3 significant digits in your answer.arrow_forwardConsider the calcination of CaCO3: CaCO3 -> CaQ + CO, Before calcination, the initial weight of CaCO3 is 2.5g. After calcination, the final weight of CaCO3 was measured at 2.0 g. Calculate the conversion of CaCO; particles (x) using the following equation: m CaCO, -m CaCo, ) x = m Caco, -MCao |arrow_forward
- 3a.arrow_forwardFor lab 4, we combined baking soda with vinegar, according to the chemical equation NaHCO 3 + CH 3COOH ==== NaCH 3COO + CO 2 + H 2O Consider 3 experiments: (i) 2g baking soda + 5mL vinegar, (ii) 2g baking soda + 10mL vinegar and (iii) 2g baking soda + 20mL vinegar. The amount of CO 2 produced was highest for (iii) and lowest for (i). For these experiments, which of the following is true? Vinegar ran out first Baking soda ran out first They both ran out at the same time Neither ran outarrow_forward00 mL of a diprotic acid primary standard solution was accurately prepared to a concentration of 0.1431 M. Three samples of this primary standard solution were used as samples in a titration to standardize an aqueous solution of sodium hydroxide, NaOH, which would be used as a titrant. Using the following table of data for the titration of the primary standard acid with NaOH, calculate the average concentration of NaOH. Trial # Volume of primary standard Initial titrant volume Final titrant volume 1 10.00 mL 8.21 mL 27.22 mL 2 10.00 mL 27.22 mL 46.23 mL 3 10.00 mL 30.28 mL 49.29 mL 0.1506 M 0.0753 M 0.0376 M 0.1431 M 0.0526 Marrow_forward
- A 500.00 mg vitamin C (MW176.12g/mol) tablet was ground, acidified, and dissolved in H20 to make a 250.0 mL solution. A 50.00 mL aliquot containing vitamin C, KI and starch was analyzed and titrated with 12.31 mL of 0.01042 M KIO3. What is the % (w/w) vitamin C in the tablet? KIO3 + 5 KI + 6 H* = 3 12 + 6 K* + 3 H20 C6H8O6 + 12 = C6H6O6 + 21 + 2H* %3D 3.39 % 33.89 % 67.77 % 22.59 %arrow_forwardHow would I calculate the number of micromoles of phosphate standard in test tubes 1-6, with the molarity of the phosphate standard being 250.0 micromoles/liter?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY