
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Calcutlate the heat of reaction for:
2NaHCO3(s)->NaCO3+CO2(g)+2H2O(1)
Expert Solution
arrow_forward
Step 1
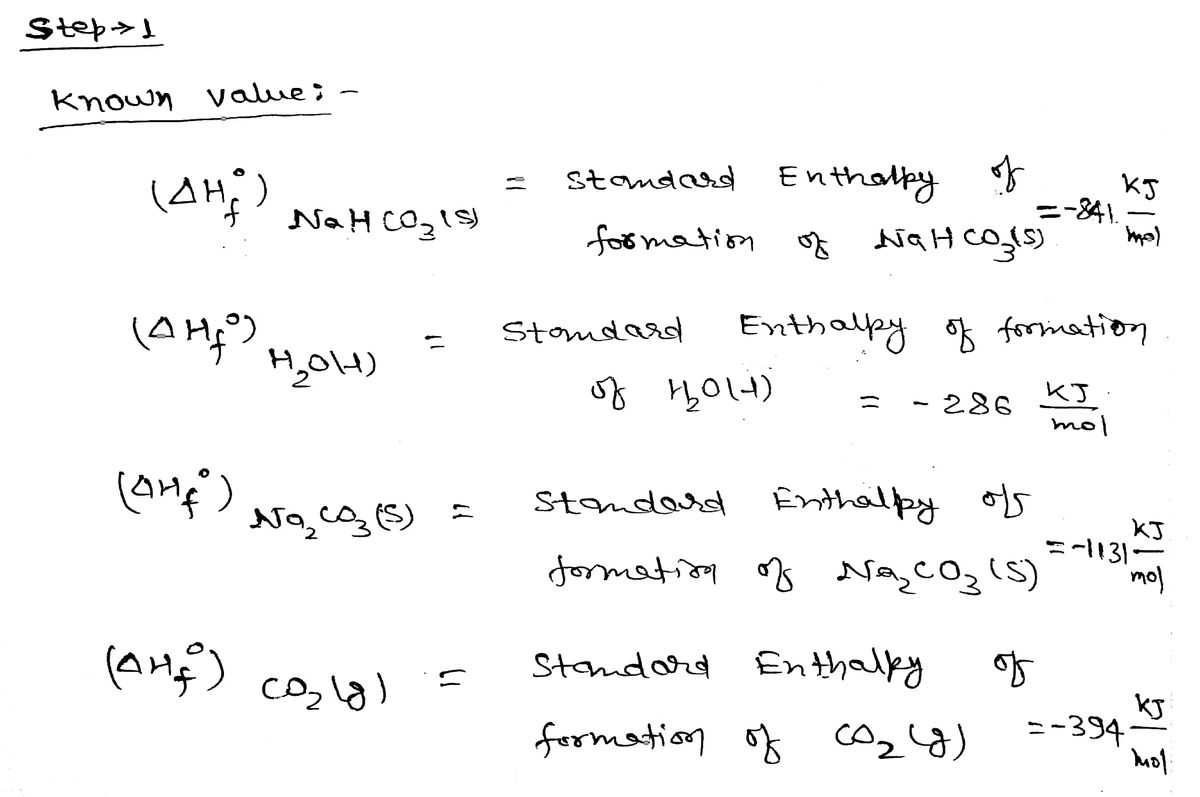
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of these reactions will not produce hydrogen gas when carried out at room temperature? (A) Li(s) + H2O(l) --> ? (B) Na(s) + H2O(l) --> ? (C) Mg(s) + H2O(l) --> ? (D) Ca(s) + H2O(l) --> ?arrow_forwardWrite expressions for Ksp for each of the ionic compounds: • • BaSO4 Mn(OH)2arrow_forward(b) oxidizes Al to Al³+ but does not oxidize Sn to Sn²+ Mg2+ Zn²+ Ca 2+ Cd²+ NO 3 Vo₂+arrow_forward
- (b) aluminum AGO = AH° = || hydride: AIH 3(S)→ Al(s) + 3/2 H₂(g) kJ/mol kJ/molarrow_forwardhydrated CuSO4 is blue in colour. why?arrow_forwardWrite a balanced chemical equation for the formation of 1 mol of Cr₂O3(s) from Cr and O2 in their standard states. (Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank. If no reaction occurs, leave all boxes blank and click on Submit.) ✓+3/20₂(g) ✓- →Cr₂O3(s) 2Cr(s) The equation of the formation of Cr₂O3 (s) from the elements: 2 Cr(s) + 3/2 O2(g) → Cr₂O3(s) b What is the enthalpy change if 3.1 g of chromium is oxidized to Cr₂O3(s)? AfH for Cr₂O3 (s) is -1134.7 kJ/mol Enthalpy change = Submit Submit Answer OCT 30 kJ Try Another Version tv ♫ 10 item attempts remaining 5 LIZA O Cengage Learning Cengage Technical Support Correct 144 B MacBook Pro * 23 Ⓡ E Previous Next Email Instructor Save and Exit Uarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY