
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
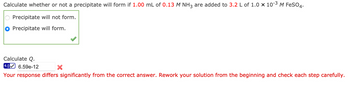
Transcribed Image Text:Calculate whether or not a precipitate will form if 1.00 mL of 0.13 M NH3 are added to 3.2 L of 1.0 × 10-³ M FeSO4.
Precipitate will not form.
Precipitate will form.
Calculate Q.
4.0 6.59e-12
Your response differs significantly from the correct answer. Rework your solution from the beginning and check each step carefully.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine emf of the cell: Fe(s) | Fe(NO3)3 (0.015M) || Zn(NO3)2 (0.025 M) | Zn(s) Standard emf Table: use values you need Fe2+ +2e Fe (s) E° = -0.44 V ---- -- Sn4+ +2 e Sn2+ EO = - 1.50 V Fe3+ Fe2+ +e ----- EO = - 0.66 V Fe3+ +3 e Fe(s) EO = - 0.68 V Mn04 + 8 H + 5 e Mn2+ + 4 H20 = - 1.27 Zn2+ + Zn (s) 2 e EO = - 0.76 V -0.111 V OA. OB. 0.331 V OC 0.163 V OD. 0.091 V + 0.080 V OE.arrow_forwardAmmonia has been studied as an alternative "clean" fuel for internal combustion engines, since its reaction with oxygen produces only nitrogen and water vapor, and in the liquid form it is easily transported. An industrial chemist studying this reaction fills a 25.0 L tank with 1.3 mol of ammonia gas and 1.9 mol of oxygen gas, and when the mixture has come to equilibrium measures the amount of water vapor to be 1.8 mol. Calculate the concentration equilibrium constant for the combustion of ammonia at the final temperature of the mixture. Round your answer to 2 significant digits. K = [] с x10 X Śarrow_forwardConsider a solution that contains just 0.535 grams of ammonium chloride in 200. mL of water (recall that ammonium is the conjugate acid to the base ammonia, NH3): a. Write an equilbrium reaction for the dissolution of aqueous ammonium chloride.arrow_forward
- 12. A solution contains 1.12 M carbonic acid. What is the concentration of the carbonate ion at equilibrium? Kat 4.4 x 107, Ka2 = 4.7 x 10¹¹1 = A. 4.4 x 107 B. 6.3 x 108 C. 1.6 x 10 D. 4.7 x 10-¹1 E. 4.2 x 10-13arrow_forward9. The following reaction was preformed in a chemistry lab 2NO(g) + Cl₂(g) = 2NOCI(g) The reaction started with 13.2 grams of NOCI in a 250.0 mL reaction vessel. At equilibrium it was determined to have 8.45 grams of NOCI. a. Determine the initial concentration of NOCI. b. C. Determine the equilibrium concentration of NOCI. Complete the ICE box for the equilibrium provided. d. Determine the equilibrium concentrations of both reactants. e. Write the Kc expression and solve for its valuearrow_forwardThe solubility of manganese carbonate, MnCOg, is 4.8 x 10-4 g/L. What is the solubility product constant for manganese carbonate? A) 2.8 × 10^-14 B) 1.7 × 10^-11 C) 3.6 × 10^-4 D) 1.3 × 10^-7 E) 3.2 × 10^-8 The third law of thermodynamics states that: A) in a spontaneous process, the entropy of the universe increases. B) there is no disorder in a perfect crystal at 0 K. C) the total energy of the universe is always increasing. D) mass and energy are conserved in all chemical reactions. E) the total energy of the universe is constant.arrow_forward
- Steam reforming of methane ( CHA) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 75.0 L tank with 34. mol of methane gas and 14. mol of water vapor, and when the mixture has come to equilibrium measures the amount of hydrogen gas to be 4.2 mol. Calculate the concentration equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant digits. K.- Explanation Check 2021 McGraw H E MacBook Air F10 F7 esc F3 F2 * # 2$ & @ 7 8arrow_forward9. Given that Agl would precipitate first than AgCl in a solution containing equimolar concentrations of I and Cl, it can be concluded that: O Ksp Agt > Ksp.AgCl O Ksp Aglarrow_forward4. Determine the equilibrium concentrations of H2C4H4O5, HC4H4O5", C4H4O,2, and H* when 3.85 M H2C4H4O6 solution achieves equilibrium. Ka1 = 1.1 x 103 and Kaz = 4.6 x a 105arrow_forward5. Given PCl5 ---> PCl3 + Cl2. 0.23 mol of PCl5 is placed in a 2.00L container. At equilibrium, 0.15mol of PCl5 is found. Calculate Keq 6. The synthesis of NH3 is given by N2 + 3H2 ---> 2NH3 all in the gas state. If the equilibrium constant is 2.73x10-8,what concentration of ammonia would be found if you began with 1.1M of each of the reactants?arrow_forwardAmmonia has been studied as an alternative "clean" fuel for internal combustion engines, since its reaction with oxygen produces only nitrogen and water vapor, and in the liquid form it is easily transported. An industrial chemist studying this reaction fills a 25.0 L tank with 5.3 mol of ammonia gas and 2.4 mol of oxygen gas, and when the mixture has come to equilibrium measures the amount of nitrogen gas to be 1.4 mol. Calculate the concentration equilibrium constant for the combustion of ammonia at the final temperature of the mixture. Round your answer to 2 significant digits. K = -0 ☐ x10 Garrow_forwardConsider the given reaction. A(aq) + B(s) C(aq) Suppose 17.3 mol A reacts with excess B in 8.75 L of solution. Calculate the concentration of C at equilibrium. tab [C] = esc ! 1 Q F 2 T K = 9.40 W w# E $ 4 R G Search or type URL % 5 T MacBook Pro 6 Y & 7 U * 8 ( ہ ۔ 9 < M O ) O - P - =arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY