
Principles of Modern Chemistry
8th Edition
ISBN: 9781305079113
Author: David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
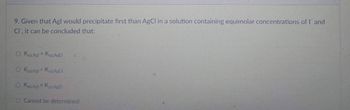
Transcribed Image Text:9. Given that Agl would precipitate first than AgCl in a solution containing equimolar concentrations of I and
Cl, it can be concluded that:
O Ksp Agt > Ksp.AgCl
O Ksp Agl<Ksp. AgCl
O Ksp.Agl = Ksp AgCl
O Cannot be determined
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Given that the Gf for Pb2+(aq) and Cl-(aq) is -24.3 kJ/mole and -131.2 kJ/mole respectively, determine the solubility product, Ksp, for PbCl2(s).arrow_forward. Under what circumstances can we compare the solubilities of two salts by directly comparing the values of their solubility products?arrow_forwardThe amino acid alanine has two isomers, -alanine and -alanine. When equal masses of these two compounds are dissolved in equal amounts of a solvent, the solution of -alanine freezes at the lowest temperature. Which form, -alanine or -alanine, has the larger equilibrium constant for ionization (HXH++X)?arrow_forward
- Describe a nonchemical system that is not in equilibrium, and explain why equilibrium has not been achieved.arrow_forwardThe following data apply to the unbalanced equation A(g)B(g) (a) On the basis of the data, balance the equation (simplest whole-number coefficients). (b) Has the system reached equilibrium? Explain.arrow_forwardThe ore cinnabar (HgS) is an important source of mercury. Cinnabar is a red solid whose solubility in water is 5.5 X 10-2 mol L-1. Calculate its \p. What is its solubility' in grams per 100 g of water?arrow_forward
- Because barium sulfate is opaque to X-rays, it is suspended in water and taken internally to make the gastrointestinal tract visible in an X-ray photograph. Although barium ion is quite toxic, barium sulfate’s /Csp of 1.1 X 10-,<) gives it such low solubility' that it can be safely consumed. What is the molar solubility' of BaSO4. What is its solubility' in grams per 100 g of water?arrow_forwardIn a particular experiment, the equilibrium constant measured for the reaction, Cl2(g)+NO2(g)Cl2NO2(g), is 2.8. Based on this measurement, calculate AG° for this reaction. Calculate AG° using data from Appendix E at the back of the book and discuss the agreement between your two calculations.arrow_forwardThe following is a thought experiment. Imagine that you put a little water in a test tube and add some NaF crystals. Immediately after you add NaF, you observe that the crystals begin dissolving. The quantity of solid NaF decreases, hut before long, it appears that no more NaF is dissolving. The solution is saturated. The equation for the dissolution of NaF in water is NaF(s) —* Na (aq) + F~(aq). As NaF dissolves, what do you think happens to the rate of dissolution? Describe w hat is occurring on the molecular level. Assume that the reverse reaction, Na+(aq) + F“(aq) —* NaF(s), also occurs as the crystal dissolves. In other words, both dissolution and precipitation are taking place. When it appears that there is no more change in the quantin’ of NaF dissolving (the solution is saturated), w hat has happened to the rates of the forward and reverse reactions? Explain your answer.arrow_forward
- Which of the systems described in Exercise 13.16 give homogeneous equilibria? Which give heterogeneous equilibria?arrow_forwardA 0.010 M solution of the weak acid HA has an osmotic pressure (see chapter on solutions and colloids) of 0.293 atm at 25 C. A 0.010 M solution of the weak acid HB has an osmotic pressure of 0.345 atm under the same conditions. (a) Which acid has the larger equilibrium constant for ionization HA[HA(aq)A(aq)+H+(aq)] or HB[HB(aq)H+(aq)+B(aq)]? (b) What are the equilibrium constants for the ionization of these acids? (Hint: Remember that each solution contains three dissolved species: the weak acid (HA or HB). the conjugate base (A- or B- and the hydrogen ion (H+). Remember that osmotic pressure (like all colligative properties) is related to the total number of solute particles. Specifically for osmotic pressure, those concentrations are described by molarities.)arrow_forwardA small quantity of a soluble salt is placed in water. Equilibrium between dissolved and undissolved salt may or may not be attained. Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning