
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Calculate the volume in milliliters of a 1.9M iron(II) bromide solution that contains 225. mmol
of iron(II) bromide (FeBr2). Round your answer to 2 significant digits.
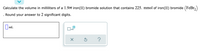
Transcribed Image Text:Calculate the volume in milliliters of a 1.9M iron(II) bromide solution that contains 225. mmol of iron(II) bromide (FeBr,)
Round your answer to 2 significant digits.
||mL
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the volume in milliliters of a 1.17M sodium chloride solution that contains 325. mmol of sodium chloride (NaCl) . Be sure your answer has the correct number of significant digits. ImL ?arrow_forwardA chemist prepares a solution of barium chloride (BaCl2) by measuring out 1.1x102 mol of barium chloride into a 500. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's barium chloride solution. Round your answer to 2 significant digits.arrow_forwardA chemist adds 475.0 mL of a 0.00126 g/L zinc oxalate (ZnC,0,) solution to a flask. Calculate the mass in milligrams of zinc oxalate the chemist has added to the flask. Be sure your answer has the correct number of significant digits. O mg Ox10arrow_forward
- Calculate the volume in liters of a 4.41x10−5μM silver(II) oxide solution that contains 175.g of silver(II) oxide AgO. Round your answer to 3 significant digits.arrow_forwardA chemist makes 640.mL of mercury(II) iodide HgI2 working solution by adding distilled water to 60.0mL of a 6.66μmolL stock solution of mercury(II) iodide in water. Calculate the concentration of the chemist's working solution. Round your answer to 3 significant digits.arrow_forwardA chemist prepares a solution of nickel(1I) chloride (NICI,) by measuring out 1.2 x 10" umol of nickel(1I) chloride into a 250. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mmol/L of the chemist's nickel(11) chloride solution, Round your answer to 2 significant digits.arrow_forward
- A chemist prepares a solution of calcium bromide (CaBr,) by measuring out 84.7 g of calcium bromide into a 150. mL volumetric flask and filling the mark with water. Calculate the concentration in mol/L of the chemist's calcium bromide solution. Round your answer to 3 significant digits. | molL Continuearrow_forwardmol A chemist makes 760. mL of potassium permanganate (KMNO,) working solution by adding distilled water to 110. mL of a 0.223 stock solution of L potassium permanganate in water. Calculate the concentration of the chemist's working solution. Round your answer to 3 significant digits. mol x10arrow_forwardA chemist must dilute 33.3 mL of 592. mM aqueous barium chlorate (Ba(ClO,) ) solution until the concentration falls to 454. mM . He'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in milliliters. Round your answer to 3 significant digits.arrow_forward
- A chemist prepares a solution of aluminum chloride (AICI3) by measuring out 56.5 g of aluminum chloride into a 300. mL volumetric flask and filling the mask to the mark with water. Calculate the concentration into mol/L of the chemist's aluminum chloride solution. Round your answer to 3 significant digitsarrow_forwardA chemist prepares a solution of barium acetate BaCH3CO22 by measuring out 2.3102mol of barium acetate into a 450.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration of the chemist's barium acetate solution. Round your answer to 2 significant digits.arrow_forwardA chemist prepares a solution of barium chloride BaCl2 by measuring out 44.0μmol of barium chloride into a 150.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in /molL of the chemist's barium chloride solution. Round your answer to 3 significant digits.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY