
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
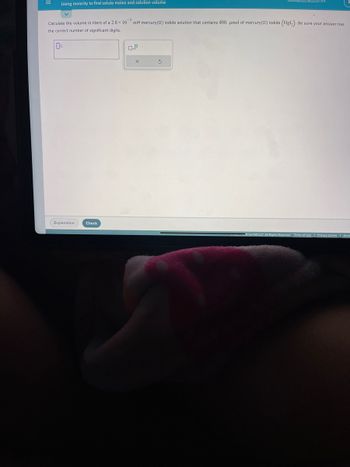
Transcribed Image Text:Using molarity to find solute moles and solution volume
-5
Calculate the volume in liters of a 2.8 x 10 mM mercury(II) iodide solution that contains 400. μmol of mercury(II) iodide (Hg1₂). Be sure your answer has
the correct number of significant digits.
Explanation Check
Xx
3
Graw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Acce
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the volume in milliliters of a 3.60 M calcium bromide solution that contains 400.mmol of calcium bromide CaBr2 . Be sure your answer has the correct number of significant digits.arrow_forwardmol L A chemist must prepare 850. mL of 2.00 M aqueous silver nitrate (AgNO3) working solution. He'll do this by pouring out some 4.47 aqueous silver nitrate stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in mL of the silver nitrate stock solution that the chemist should pour out. Be sure your answer has the correct number of significant digits.arrow_forwardA chemist prepares a solution of calcium bromide (CaBr₂) by measuring out 0.599 kg of calcium bromide into a 400. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's calcium bromide solution. Be sure your answer has the correct number of significant digits. e mol/L Xx 扁 olarrow_forward
- A chemist adds 305.0 mL of a 0.41 M barium chloride (BaCl₂) solution to a reaction flask. Calculate the millimoles of barium chloride the chemist has added to the flask. Round your answer to 2 significant digits. 1.25 x 10 mmol [NN] X 5 ? Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Ad DII F11 F12 F10 FO FB F7 esc ! 1 Explanation 2 Check 39: F2 14/ # 3 80 F3 $ 4 000 F4 R % 5 ܐ FS T ^ 6 F6 Y & 7 U * 00 8 ہ ۔ 9 ) 0 0 P + 11 T D olo Ararrow_forwardA chemist prepares a solution of zinc nitrate (Zn(NO,).) by weighing out 166. g of zinc nitrate into a 300. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in g/L of the chemist's zinc nitrate solution. Be sure your answer has the correct number of significant digits. x10arrow_forwardA chemist makes 410. mL of magnesium fluoride (MgF₂) working solution by adding distilled water to 280. mL of a mol 0.00188 stock solution of magnesium fluoride in water. L Calculate the concentration of the chemist's working solution. Round your answer to 3 significant digits. mol L X 5 F 00 18 Ararrow_forward
- A chemist must dilute 88.2mL of 6.18mM aqueous copper(II) fluoride CuF2 solution until the concentration falls to 5.00mM. She'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in liters. Be sure your answer has the correct number of significant digits.arrow_forwardA chemist prepares a solution of calcium bromide (CaBr₂) by measuring out 470. g of calcium bromide into a 350. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's calcium bromide solution. Round your answer to 3 significant digits. mol/L X 5arrow_forwardA chemist prepares a solution of sodium thiosulfate (Na₂S₂O3) by measuring out 113. g of sodium thiosulfate into a 150. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's sodium thiosulfate solution. Be sure your answer has the correct number of significant digits.arrow_forward
- The chemical formula for hydrogen bromide is HBr. A chemist measured the amount of hydrogen bromide produced during an experiment. She finds that 5.9 g of hydrogen bromide is produced. Calculate the number of moles of hydrogen bromide produced. Be sure your answer has the correct number of significant digits. 477. mol x10 X S 4arrow_forwardA chemist prepares a solution of calcium sulfate (CaSO4) by measuring out 0.28 g of calcium sulfate into a 250. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's calcium sulfate solution. Be sure your answer has the correct number of significant digits. E 0 10⁰ × x 10 mol/L 19 x10 C 6arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY