
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Question 14 of 14
Submit
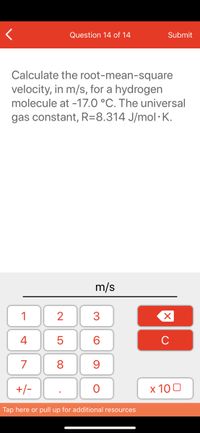
Calculate the root-mean-square
velocity, in m/s, for a hydrogen
molecule at -17.0 °C. The universal
gas constant, R=8.314 J/mol· K.
m/s
1
2
3
4
6.
C
7
8
+/-
x 10 0
Tap here or pull up for additional resources
LO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following situation: You have a 50 L tank of compressed air at 15 atm and 100 ºC. You then pass the compressed air through a "titanium getter" which contains fragments of hot titanium metal. The purpose of the "getter" is to remove oxygen gas from the sample as Ti reacts with oxygen to form solid TiO2 (known as titania). Once the purified air is passed through the getter is it cooled back down to 100 ºC and fills another 50 L storage tank. If air typically contains about 20% oxygen, what could the pressure in the storage tank be? Question 2 options: A) 12 atm B) 15 atm C) 18 atm D) 24 atmarrow_forwardFor many purposes we can treat ammonia (NH3) a as an ideal gas at temperatures above its boiling point of -33. °C. 3 Suppose the pressure on a 1.0 m sample of ammonia gas at -2.00°C is reduced to one-third its initial value. Is it possible to change the temperature of the ammonia at the same time such that the volume of the gas doesn't change? If you answered yes, calculate the new temperature of the gas. Round your answer to the nearest °C. yes no °C x10 Sarrow_forward1. Express R, the gas law constant in units of mL•kPa/mol•K by converting L and atm to mL and kPa, respectively. Show all your work and calculations.arrow_forward
- A sample of 3.42 mol of xenon is confined at low pressure in a volume at a temperature of 86 °C. Describe quantitatively the effects of each of the following changes on the pressure, the average kinetic energy per molecule in the gas, and the root-mean-square speed. (a) The temperature is increased to 199 °C (b) The volume is tripled. (c) The amount of xenon is decreased to 1.87 mol Give each answer as a decimal factor of the form: new value factor old value. A factor of 1 means no change. ChangeP KEavgmsarrow_forwardAn experiment is performed to determine the volume of gas produced by the following reaction: 2HCl(aq) + Mg(s) MgCl2(aq) + H2(g) A. What is the maximum mass of Mg in g that could be reacted at 20oC temperature and 1.0atm, if the volume of the gas burette is 50mL? (Hint: Find moles of H2 using the ideal gas law; then, convert to g of Mg.) B. If the experiment were moved to a higher elevation and the pressure was decreased to 0.95 atm would the maximum amount of Mg increase or decrease? C. If 16mg of Mg were reacted, what is the volume of gas produced in this reaction? D. What is the solvent for this reaction? E. Would the HCl(aq) be considered a strong, weak, or non-electrolyte? F. Would the MgCl2(aq) be considered a strong, weak, or non-electrolyte?arrow_forwardA 1.00 LL flask is filled with 1.20 gg of argon at 25 ∘C∘C. A sample of ethane vapor is added to the same flask until the total pressure is 1.350 atmatm . part A: What is the partial pressure of argon, PArPAr Part B: What is the partial pressure of ethane, PethanePethanearrow_forward
- A particular balloon can be stretched to a maximum surface area of 1257 cm². The balloon is filled with 3.2 L of helium gas at a pressure of 759 torr and a temperature of 310 K. The balloon is then allowed to rise in the atmosphere. Assume an atmospheric temperature of 273 K and determine at what pressure the balloon will burst. (Assume the balloon to be in the shape of a sphere.) Express your answer using two significant figures. 15] ΑΣΦ P = 0.22 Submit Previous Answers Request Answer ? X Incorrect; Try Again; 5 attempts remaining atmarrow_forwardThe van der Waals constants for HCl are a = 3.67 atm·liter2·mole–2, and b = 40.8 cc·mole–1. Find the critical constants of this substance.arrow_forwardWhen limestone (solid CaCO3) is heated, it decomposes into lime (solid CaO) and carbon dioxide gas. This is an extremely useful industrial process of great antiquity, because powdered lime mixed with water is the basis for mortar and concrete - the lime absorbs CO₂ from the air and turns back into hard, durable limestone. Suppose a limekiln of volume 800. L is pressurized with carbon dioxide gas to 14.9 atm, and heated to 960.0 °C. When the amount of CO, has stopped changing, it is found that 3.96 kg of CaCO, have appeared. Calculate the pressure equilibrium constant K, this experiment suggests for the equilibrium between CaCO3 and CaO at 960.0 °C. Round your answer to 2 significant digits. Note for advanced students: it's possible there was some error in this experiment, and the value it suggests for K, does not match the accepted value. K = 3.07 Parrow_forward
- When limestone (solid CaCO3) is heated, it decomposes into lime (solid CaO) and carbon dioxide gas. This is an extremely useful industrial process of great antiquity, because powdered lime mixed with water is the basis for mortar and concrete - the lime absorbs CO₂ from the air and turns back into hard, durable limestone. Suppose some calcium carbonate is sealed into a limekiln of volume 550. L and heated to 520.0 °C. When the amount of CaCO3 has stopped changing, it is found that 8.46 kg have disappeared. Calculate the pressure equilibrium constant K, this experiment suggests for the equilibrium between CaCO3 and CaO at 520.0 °C. Round your answer to 2 significant digits. P Note for advanced students: it's possible there was some error in this experiment, and the value it suggests for K does not match the accepted value. 0 Xarrow_forwardThe air pollutant NO is produced in automobile engines from the high-temperature reaction: N₂(g) + O2(g) = 2 NO(g) K 1.7 x 103 at 2300 K.arrow_forwardWhen limestone (solid CaCO3) is heated, it decomposes into lime (solid CaO) and carbon dioxide gas. This is an extremely useful industrial process of great antiquity, because powdered lime mixed with water is the basis for mortar and concrete the lime absorbs CO₂ from the air and turns back into hard, durable 2 limestone. Suppose some calcium carbonate is sealed into a limekiln of volume 400. L and heated to 740.0 °C. When the amount of CaCO3 has stopped changing, it is found that 3.37 kg have disappeared. P 00. Calculate the pressure equilibrium constant K this experiment suggests for the equilibrium between CaCO3 and CaO at 740.0 °C. Round your answer to 2 significant digits. Ar Note for advanced students: it's possible there was some error in this experiment, and the value it suggests for K does not match the accepted value. р K₁ = 0 x10 р x Ś ? Explanation Check 0 81 K © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY