
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:Leaming
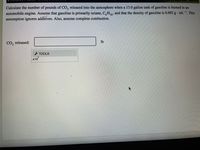
Calculate the number of pounds of CO, released into the atmosphere when a 13.0 gallon tank of gasoline is burned in an
automobile engine. Assume that gasoline is primarily octane, C,H,13, and that the density of gasoline is 0.692 g · mL-. This
assumption ignores additives. Also, assume complete combustion.
CO, released:
lb
- TOOLS
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- #12arrow_forwardEthanol, C2H6O, is most often blended with gasoline - usually as a 10 percent mix - to create a fuel called gasohol. Ethanol is a renewable resource and ethanol-blended fuels, like gasohol, appear to burn more efficiently in combustion engines. The heat of combustion of ethanol is 326.7 kcal/mol.The heat of combustion of 2-methylhexane, C7H16, is 1.150×103 kcal/mol. How much energy is released during the complete combustion of 446 grams of 2-methylhexane ?________ kcal Assuming the same efficiency, would 446 grams of ethanol provide more, less, or the same amount of energy as 446 grams of 2-methylhexane? _________. (more, less, or the same amount)arrow_forwardDUE NOW. Please answer it neatly and with DETAILED solutions. Thanks!arrow_forward
- Given the reaction: 2 Na(s) + Cl2(g) → 2 NaCI(s) Which statement is true? O The conversion factor for chlorine gas to sodium chloride is: 1 mol Cl2 is equivalent to 2 mol NaCI O The conversion factor for chlorine gas to sodium chloride is: 2 mol Cl is equivalent to 2 mol NaCl O The conversion factor for sodium metal to sodium chloride is: 1 mol Na is equivalent to 2 mol NaCl O The conversion factor for sodium metal to sodium chloride is: 2 mol Na is equivalent to 1 mol NaCIarrow_forward7. Applying the principles of green chemistry requires some consideration of the way in which they can be applied. Figure 1 represents a generic industrial process. Using the principles of green chemistry and circled letters, describę how four of the principles could be applied to this process to make it a green operation. E kaEA product raw materials energy Source waste гeactor Figure 1 starrow_forwardCommercial concentrated hydrochloric acid has the following specification: Density = 1.2 g/mL; Weight percentage is 37% w/w: Molecular weight of HCl is 36.46 g/mol. If 12.0 mL of conc HC) is added to the reaction, how many moles of HCI have been added? Report your answer to the correct number of significant figures: do not report the units in your answer.OGMU 2020 QUESTION 3 If 5.0 mL of t-pentyl alcohol are involved in the reaction, how many moles of t-pentyl alcohol are present? Round your answer to the correct number of significant figures. Do not include the units in your answer. IUPAC name of t-pentyl alcohol is 2-methyl-2-butanol.OGMU 2020 (Hint: use the CRC Handbook Of Chemistry and Physics from our online library to get the correct numerical constants.) QUESTION 4 Given that the nucleophilic substitution reaction used 5.0 mL of t-pentyl alcohol and 12.0 mL of conc. hydrochloric acid to produce t-pentyl chloride, what was the limiting reagent in the overall reaction? GMU 2020 O…arrow_forward
- Acetylene torches are used for welding. These torches use a mixture of acetylene gas, C2H2C2H2, and oxygen gas, O2O2 to produce the following combustion reaction: 2C2H2(g)+5O2(g)→4CO2(g)+2H2O(g)2C2H2(g)+5O2(g)→4CO2(g)+2H2O(g) Part A Imagine that you have a 7.00 LL gas tank and a 4.50 LL gas tank. You need to fill one tank with oxygen and the other with acetylene to use in conjunction with your welding torch. If you fill the larger tank with oxygen to a pressure of 125 atmatm , to what pressure should you fill the acetylene tank to ensure that you run out of each gas at the same time? Assume ideal behavior for all gases. Express your answer with the appropriate units.arrow_forwardOne commercial system removes SO2 emissions from smoke at 95.0°C by the following set of balanced reactions: SO2(g) + Cl2 → SO2Cl2(g) SO2Cl2 + 2H2O → H2SO4 + 2HCl H2SO4 + Ca(OH)2 → CaSO4(s) + 2H2O Assuming the process is 0.86 % efficient, how many grams of CaSO4 may be produced from 91 g of SO2? Report your answer to 3 sig figs.arrow_forwardCould someone please help me with this? No Plagirisim Please! surroundings – everything outside of the system being studied system – the reactants and products in a chemical reaction'' 1 · What comes to mind when you think of the terms “system” and “surroundings”? Provide 2-3 examples of each. Unit 2 lesson 3 2 What are some ways a system can be impacted by its surroundings?arrow_forward
- Hydrocarbons are compounds that contain only C and H atoms. When a hydrocarbon reacts with O2, CO2 and H2O are formed. Write a balanced equation for the combustion of the following hydrocarbon, a high-octane component of gasoline. Do not include states of matter in your answer. C6H6 (benzene)arrow_forwardMaking Calcium Sulfate: MgSO4 (aq) + CaCl2 (aq) -------> CaSO4 (s) + MgCl2 (aq) Given Data: mass of calcium chloride = 1.00grams, mass of magnesium sulfate = 1.00 grams, mass of Calcium Sulfate=0.70grams With the Given data, find out the limiting reactant, theortical yeild, and percentage yield of calcium sulfate?arrow_forward✓ 个 < app.101edu.co STARTING AMOUNT X How many grams of oxygen gas are produced when 2.43 x 104 g of KCIO, are completely reacted according to the following chemical equation: 3.5 1 ADD FACTOR * ( ) 32.00 9.52 x 10-5 g KCIO3 2 KCIO3(s) → 1.90 x 10-4 Question 31 of 38 2 mol KCIO3 O 3 2 KCI(S) + 3 M 74.55 mol KCI ANSWER 31 1.98 x 10-6 5.95 x 10-6 mol O₂ O₂(g) 2.43 x 104 6.022 x 10²3 g 0₂ by RESET 2 2.97 x 10-6 122.55 g KCI Feb 23 7:11 Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY