
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Calculate the heat of reaction of the following reactions. Please note that the reaction coefficients may not be balanced.
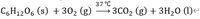
Transcribed Image Text:C6H₁2O6 (s) + 30₂ (g)
12
37 °C
→ 3CO₂ (g) + 3H₂0 (1)<
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Which of the following term/s is/are true about tacticity? i. Tacticity affects the degree of crystallinity. ii. Tacticity is a polymer property that occurs as a result of chain length and chain structure. iiI. An isotactic polymer shows greater resistance to dissolve in solvents than its atactic and syndiotactic forms. iv. An atactic polymer is melted at higher temperatures than its isotactic and syndiotactic forms. v. When an isotactic polymer is melted, it shows higher melt viscosity than its atactic and syndiotactic forms. I, II, II, Nand V A I, Ill and V III, VandV NandVarrow_forwardplease type out or diagrams so that it is easy to readarrow_forwardExplain what the difference is between a strong and a weak acid. Give an example of each?arrow_forward
- READ THE INSTRUCTIONS!!! DO NOT ANSWER THIS IF YOU ALREADY ANSWERED. I WILL DOWNVOTE. ANSWER THIS COMPLETE, CORRECT, TYPEWRITTEN. I WANT IT TO BE DIGITALLY ANSWERED NOT HANDWRITTEN. I WILL UPVOTE IF YOU MEET THESE.arrow_forwardLiquid phase sintering is used for WC–Co compacts, even though the sintering temperatures are below the melting points of either WC or Co. How is this possible?arrow_forwardResearch for the boiling point of the following mixture of compounds. Encircle which would boil first. Finally, indicate what type of distillation will you use to separate them.arrow_forward
- Write a technical report in no more than five pages on Potash processing using hot leach process and cold crystallization process as: 1- Describe the impact of the following on the hot leach process: a. solar pans, mother liquor loop, how does crystallization of KCI occur in this plant and what happens to the pressure in these crystallizers. 2- Describe the technical operations in each step of the cold crystallization. 3- Compare both processes in terms advantages and disadvantages.arrow_forwardWhat are reaction intermediates? Explain stability, preparation and reactivity of carbocation, carbanion and free radicals.arrow_forwardAnswer the following question using the reaction coordináte diagram shown below. 280 240 E 200- E 160- 120+ 80 40- Time What is the enthalpy (AH) of the reverse reaction? A. 240 kJ B. -80 kJ C. 80 kJ D. 160 kJ Heat content (H) kilojoulesarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The