
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
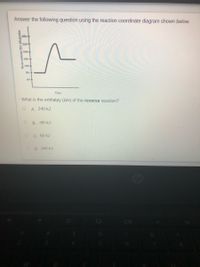
Transcribed Image Text:Answer the following question using the reaction coordináte diagram shown below.
280
240
E 200-
E 160-
120+
80
40-
Time
What is the enthalpy (AH) of the reverse reaction?
A. 240 kJ
B. -80 kJ
C. 80 kJ
D. 160 kJ
Heat content (H) kilojoules
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Given the reaction C,H16+11 O2→7 CO2+8 H20 determine the extent of reaction if 100 g mol of O2 enter a reactor, and 8 g mol of O, leave the reactor. Also determine the amount of water formed.arrow_forwardExample: consider the shock-heating of air to 2500K and 3atm. Find: 1. The initial nitric oxide formation rate in ppm/s. 2. The amount of nitric oxide formed (in ppm)in 0.25ms. Solution: From table 3.3 Kp=0.0002063arrow_forwardPlease I need solutions to this past question,I need it before 8:00pm WSTarrow_forward
- The reaction specific heat is -3489.63. This reaction is self-sustaining or not? Why?arrow_forwardSubmit Question 27 of 30 Electrolyzing molten NaCl produces what products at the cathode and anode, respectively? A) Na(l), Cl:(g) B) Cl:(g), Na(l) C) Na*(aq), Cl (aq) D) CI (aq), Na (aq) E) Na(l), CI(I) 8:35 PM 11/22/2020 13 Type here to search End F10 PgUp Fm Prt Scn F8 Home F9 D FS F6 DII F3 F4 &arrow_forward1 Q5// For the adiabatic reaction a A+bBcC+d D with constant heat capacities, show that the reaction temperature as a function of conversion is given by: T=To+ (-AH) X ΣOCRarrow_forward
- Q (6) An aqueous phase reversible reaction RS is carried out between 0°C and 100°C. free energy of reaction and heat of reaction at 298K is, AH, 298K-20000 cal/gm mole A298K-3510 cal/gm mole Calculate: (i)Equilibrium constant at both temperatures (0°C and 100°C) (ii)Equilibrium conversiont at both temperatures (0°C and 100°C)C) (iii) In the above problem, If the fractional conversion needs to be 80% or more, what should be minimum temperature level in the reactor? (Value of universal gas constant R= 1.987 cal/gm mole.)arrow_forwardThe activation energies for self-diffusion of tungsten (W), nickel (Ni) and lead (Pb) are640, 280 and 110 kJ/mol. Show that the relationship, Q ~ 20 R Tm, is a goodapproximation. Here R is the universal gas constant and Tm is the melting point.arrow_forwardWhich of the following is true about wood pellets? O 1+2 O 3- High Cost O 2- Has the best combustion properties O All of these O 1- The densest form of solid biomassarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The