
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
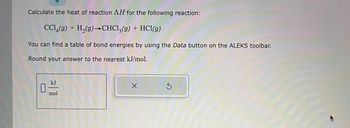
Transcribed Image Text:Calculate the heat of reaction AH for the following reaction:
CC14(g) + H₂(g) →CHCl3(g) + HCl(g)
You can find a table of bond energies by using the Data button on the ALEKS toolbar.
Round your answer to the nearest kJ/mol.
1
kJ
mol
X
S
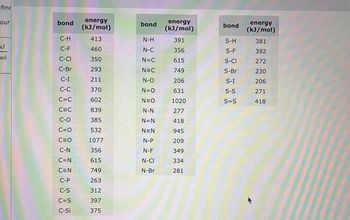
Transcribed Image Text:find
your
kJ
nol
bond
C-H
C-F
C-CI
C-Br
C-I
C-C
C=C
C=C
C-O
C=O
C=O
C-N
C=N
C=N
C-P
C-S
C=S
C-Si
energy
(kJ/mol)
413
460
350
293
211
370
602
839
385
532
1077
356
615
749
263
312
397
375
bond
N-H
N-C
N=C
N=C
N-O
N=O
N=O
N-N
N=N
N=N
N-P
N-F
N-CI
N-Br
energy
(kJ/mol)
391
356
615
749
206
631
1020
277
418
945
209
349
334
281
bond
S-H
S-F
S-CI
S-Br
S-I
S-S
S=S
energy
(kJ/mol)
381
392
272
230
206
271
418
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using bond energies determine the heat generated when 32 grams of methane, CH4, is burned. (in kJ) CH4 + O2 → CO2 + H2Oarrow_forwardWhat is the enthalpy change when 4.70 grams of HF(g) (molar mass= 20.0 g/mol) are formed by the following reaction? H2(g) + F2(g) ⟶ 2HF(g) ΔH = -537 kJarrow_forwardUsing bond energies determine the heat generated when 32 grams of methane, CH4, is burned. (in kJ) CH4 + O2 → CO2 + H2Oarrow_forward
- 2 pictures to show bond energiesarrow_forwardCalculate the standard enthalpy of reaction for the combustion of methane. Round to the nearest whole number.CH4(g) + O2 --> CO2(g) + 2H2O(l) kJ/mol Compound Hf (kJ/mole) CH4(g) -75 CO2(g) -394 H2O(l) -284 This reaction is: endothermic or exothermicarrow_forwardCalculate the heat of reaction AH for the following reaction: 2 HCl(g) + Br,(g)→2 HBr(g) + Cl,(g) You can find a table of bond energies by using the Data button on the ALEKS toolbar. Round your answer to the nearest kJ/mol. kJ ? molarrow_forward
- Question 7. Use the bond energies provided to estimate ΔH°rxn for the reaction below. CH3OH(l) + 2 O2(g) → CO2(g) + 2 H2O(g) ΔH°rxn = ? Bond Bond Energy (kJ/mol) C-H 410 C-O 359 C=O 799 O=O 498 O-H 454arrow_forwardPredict the enthalpy change for the reaction below using the thermochemical equations provided. 2 C2H2 + 5 O2 --> 4 CO2 + 2 H2O 4 C + 2 H2 --> 2 C2H2 Δ Hrxn = + 226.7 kJ/mol C + O2 --> CO2 Δ Hrxn = - 393.5 kJ/mol 2 H2 + O2 --> 2 H2O Δ Hrxn = - 571.6 kJ/mol Group of answer choices -2372 kJ/mol -1192 kJ/mol -1919 kJ/mol -738.4 kJ/molarrow_forwardCalculate the heat of reaction AH for the following reaction: 2 CH4(9) + 30₂(g) 2 CO(g) + 4H₂O(g) You can find a table of bond energies by using the Data button on the ALEKS toolbar. Round your answer to the nearest kJ/mol. 0 kJ mol X Śarrow_forward
- Nitesharrow_forwardCalculate the heat of reaction AH for the following reaction: CH4(g) + 202(g) →CO2(g) + 2H2O(g) You can find a table of bond energies by using the Data button on the ALEKS toolbar. Round your answer to the nearest kJ/mol. mol Garrow_forward! 1 Q :0 F1 Calculate the enthalpy of the reaction below (AHrxn, in kJ) using the bond energies provided. 2 H₂(g) + O₂(g) → 2 H₂O(g). 482 133,809 (a 2 W F2 # 3 E Single Bond M Multiple Bonds I U ZO 80 F3 602 C=O 799 835 C=O 1072 C=N 615 O=O 494 C=N 887 N=N 942 **All values in kJ/mol** oc: 7 11 $ 4 H 432 411 346 386 305 167 459 C=C C=C O CNO a F4 358 201 142 SD F % 5 tv 0 F5 6 Q G F6 NA & 7 F7 G H 33611 RTY U * 8 W F8 ( 9 F9arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY